Evaluation of airway resistance in primary small cell carcinoma of the trachea by MostGraph: a case study
Introduction
Primary malignant tumors of the trachea are rare, accounting for 0.1% of respiratory malignancies (1). The most common histological types of tracheal malignancies include squamous cell carcinoma and adenoid cystic carcinoma; to date, small cell lung cancer (SCLC) continues to have an extremely low incidence and few reported cases (2,3). We report the case of a patient who we treated for SCLC that caused tracheal stenosis. Symptoms of primary tracheal tumors are difficult to detect, unless >70% of the bronchus is obstructed; in several instances, its early stages may be difficult to differentiate from other causes of airway obstruction, such as chronic obstructive pulmonary disease and asthma.
In clinical practice, airway obstruction may be differentiated as upper or lower, or central or peripheral, by the flow volume (FV) curve on spirometry and airway resistance by a forced oscillation technique, MostGraph. Here we compared both tests before and after resection of a primary SCLC of the trachea that caused upper airway obstruction. Furthermore, we discussed the significance of these measurements in upper airway stenosis caused by SCLC.
Case presentation
A 58-year-old woman, with no particular medical history, consulted our hospital with a chief complaint of respiratory discomfort. She is a current smoker of 20 cigarettes per day for 40 years; she has no drinking habit. Her father had diabetes and mother had hypertension.
Two months ago, the patient began to experience respiratory discomfort upon exertion and consulted her local physician. On suspicion of bronchial asthma, oral theophylline and long-acting β2 agonist were prescribed; however, her symptoms failed to improve and progressed to respiratory discomfort and wheezing at rest. Therefore, the patient was referred to our hospital.
On initial physical examination, she was observed to have a body temperature of 35.6 °C, blood pressure of 126/66 mmHg, heart rate of 86 beats per min, respiratory rate of 21 breaths per min, oxygen saturation (SpO2) of 98% at room air, and no tumor was palpated on the neck. On auscultation, wheezing was heard on both sides of the chest.
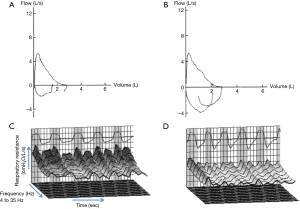
Initial tests revealed elevated serum progastrin-releasing peptide, a tumor marker, at 108 mg/dL; carcinoembryonic antigen and neuron-specific enolase levels were normal. No abnormal findings were observed in other blood tests. Chest radiograph was normal. Pulmonary function testing revealed peripheral airway obstruction on the FV curve (Figure 1). Pulmonary function test demonstrates airflow obstruction, with forced expiratory volume in one second (FEV1) of 2.02 L and FEV1% of 68.9%, forced expiratory flow rates (FEF25–75) of 1.10 L/sec. There are no restrictive ventilatory defects, vital capacity (VC) of 2.93 L, %VC of 116.3%, forced vital capacity (FVC) of 2.93 L and %FVC of 116.3%. Spirometry was performed by using the CHESTAC-8800 (Chest, Tokyo, Japan). All pulmonary function tests were performed as recommended by the American Thoracic Society (ATS)/European Respiratory Society (ERS). Fractional exhaled nitric oxide (FeNO) test was normal, with FeNO level of 5 ppb.
The wheezing heard in both lungs on initial examination was attributed to bronchial asthma; therefore, treatment with inhaled fluticasone propionate/formoterol fumarate and oral montelukast was initiated. However, 1 week later, there was no improvement in the respiratory discomfort and intensified wheezing was observed in the neck area. Upper airway obstruction was suspected, and chest CT was performed, revealing a 13-mm irregular mass protruding into the tracheal cavity, with tracheal stenosis of approximately 60–70%. Enlargement of the left paratracheal lymph node near the tumor was observed (Figure 2A,B).

Because of the high risk of airway obstruction, tracheotomy and tracheal tumor resection were performed at day 3 of hospitalization. Postoperative bronchoscopy revealed no visible residual tumor. Pathological findings of the resected specimen demonstrated invasion of atypical cells with abundant nuclear chromatin and irregular nuclei, consistent with SCLC (Figure 3). The patient was designated to be stage IIIB (T4N2M0). According to the Japanese clinical guideline for the treatment of SCLC, two courses of cisplatin plus etoposide chemotherapy were administered, followed by radiation therapy (total dose of 50 Gy), which the patient tolerated. Because the original lesion did not recur and the left paratracheal lymph node reduced in size, the therapeutic outcome was deemed to be a complete response.

Chest CT images and pulmonary function tests from before and after therapy were compared, revealing no major change in the FV curve (Figure 1A,B); however, there was almost no change in FVC, %FVC, FEV1, FEV1% and FEF25–75 in spirometry. Pre-treatment MostGraph results were R5 5.58 cmH2O/L/s, R20 3.94 cmH2O/L/s, R5–R20 1.64 cmH2O/L/s, X5 −3.30 cmH2O/L/s, Fres 19.63 Hz, and low-frequency reactance area (AX) 24.95 cmH2O/L; whereas post-treatment results revealed a marked improvement of R5 3.75 cmH2O/L/s, R20 3.21 cmH2O/L/s, R5–R20 0.54 cmH2O/L/s, respiratory system reactance (Xrs) at 5 Hz −0.41 cmH2O/L/s, frequency of resonance (Fres) 7.25 Hz, and AX 1.30 cmH2O/L (Figure 1C,D).
Discussion
Among primary neuroendocrine tumors of the trachea, carcinoid is the most common, whereas SCLC is extremely rare, with only a handful of cases reported to date (2,3). In general, tumors of the trachea most commonly develop in the lower portion (4); in our patient, the tumor was limited to the upper trachea, which is a rare case.
Typical symptoms of tracheal tumors are nonspecific and generally include coughing, shortness of breath, wheezing, respiratory discomfort, and bloody phlegm (5). In addition, tracheal tumors generally exhibit few symptoms until the size exceeds two-thirds of the tracheal cavity; therefore, early stage cases may be easily overlooked and diagnosis is often delayed (1,6). On initial examination, tracheal tumors are often misdiagnosed and treated as chronic bronchitis, bronchial asthma, chronic obstructive pulmonary disease (COPD), and other conditions. As observed in our patient, the tumor is often advanced by the time it manifests with respiratory discomfort and wheezing; in several cases, there is a high risk of airway obstruction during diagnosis. Based on several previous cases in which bronchial asthma was initially diagnosed from symptoms and treated accordingly, we suggest that upper airway obstruction (i.e., tracheal tumor) must be considered when response to bronchodilator treatment is poor and if stridor on inhalation is heard on auscultation.
In the differential diagnosis of upper airway obstruction, the characteristic FV curve on pulmonary function test is useful. However, our patient did not exhibit the characteristic trapezoid FV curve. In addition, there was no change in the FEV1 and FV curve following tumor resection. We deduce that the sensitivity of FV curve pattern for detection to upper airway obstruction, would depend on the nature of the tumor, such as hardness, airway site, pedunculated tumor. In our patient, FEV1, FEV 1.0%, and FEF25–75 were persistently low and airflow obstruction was irreversible despite administration of bronchodilators and tumor resection. In addition, with low FEF25–75, and the patient’s smoking history of >10 packs a year, we believe that these results on pulmonary function testing may be attributable to concurrent COPD and do not reflect upper airway obstruction.
Although there was no change in the FEV1 following tumor resection, R5, R20, Fres (indicator for the elastic or inertial properties of the lung), and ALX (indicator for the elastic forces) were markedly decreased; these changes can be interpreted as caused by upper airway obstruction (6). MostGraph-01 is an equipment to measure airway resistance and respiratory reactance. Rrs indicate comprehensive viscous resistance of respiratory system such as airway resistance and pulmonary tissue resistance. Meanwhile, Xrs is an indicator for elasticity of the respiratory system. Although the relationship between the changes of these indicators measured by the MostGraph-01 and pathological changes in lung diseases is still controversial, MostGraph is considered to be a good indicator on the evaluation for the structural change in the airways. To evaluate the soft pedunculated tumor like this case, MostGraph is thought to be more useful than spirometry, because MostGraph-01 measures the airway resistance using multiple oscillation frequencies under resting breathing.
Conclusions
We encountered a case of primary tracheal SCLC that was initially diagnosed as bronchial asthma, based on pulmonary function testing. Because there was no response to initial treatment, further work-up was pursued and resulted in definitive diagnosis and treatment of a tracheal tumor. For cases that are treated as bronchial asthma, but do not respond to treatment and present with wheezing on inhalation, we believe that suspecting upper airway obstruction is important. Radiological imaging, FV curve on pulmonary function test, and MostGraph-01 are useful for a thorough differential diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: Y Gon received honoraria from Nippon Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Astellas Pharma, and Novartis Pharmaceuticals. S Hashimoto received honoraria from Nippon Boehringer Ingelheim, GlaxoSmithKline, Kyorin Pharmaceutical, AstraZeneca, Astellas Pharma, Novartis Pharmaceuticals, Abbott Japan, and MSD. Y Gon, S Maruoka and S Hashimoto received research funding from Nippon Boehringer Ingelheim, GlaxoSmithKline, Kyorin Pharmaceutical, AstraZeneca, Astellas Pharma, Novartis Pharmaceuticals, and Pfizer Japan. The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Qiu J, Lin W, Zhou ML, et al. Primary small cell cancer of cervical trachea: a case report and literature review. Int J Clin Exp Pathol 2015;8:7488-93. [PubMed]
- Heikal M. Small-cell cancer presenting as a tracheal polyp: a case report and review of the literature. J Bronchology Interv Pulmonol 2012;19:132-6. [Crossref] [PubMed]
- Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol 2011;34:32-7. [Crossref] [PubMed]
- Takano S, Kusumoto M, Tateishi U, et al. A case of primary small cell carcinoma in the trachea of a young man. Haigan 2005;45:133-7. [Crossref]
- Jain S, Agarwal JP, Gupta T, et al. Case report: Second primary small cell carcinoma of the trachea in a breast cancer survivor: a case report and literature review. Br J Radiol 2008;81:e120-2. [Crossref] [PubMed]
- Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003;22:1026-41. [Crossref] [PubMed]


