Suberoylanilide hydroxamic acid attenuates paraquat-induced pulmonary fibrosis by preventing Smad7 from deacetylation in rats
Introduction
Paraquat (PQ) is a widely used herbicide worldwide and causes pulmonary fibrosis by long-term accumulation or acute intoxication (1-4). Pulmonary fibrosis is characterized by excessively synthesizing and accumulating extracellular matrix proteins, thus resulting in irreversible loss of lung function. The general pathological feature of pulmonary fibrosis includes: (I) the excessive deposition of collagen; (II) fibroblast differentiation into myofibroblasts, which are major collagen-producing cells when activated, and characterized by expressing α-smooth muscle actin (α-SMA). Excessive collagen deposition destroys the architecture of the normal lung parenchyma and results in respiratory failure. Despite advanced progress made in understanding the pathogenesis of lung fibrosis, effective therapy for lung fibrosis is still limited and the prognosis remains poor. Therefore, understanding molecular mechanism of lung fibrosis is critical to provide novel therapeutic targets for developing effective antifibrotic drugs.
In lung fibrogenesis, transforming growth factor-β1 (TGF-β1)/Smad signaling pathway plays an essential role by promoting fibroblasts differentiation into myofibroblasts and the epithelial to mesenchymal transition (EMT) (5,6). Upon activation, TGF-β1 receptors I (TβRI) phosphorylate Smad2 and Smad3. Therefore blocking TGF-β1/Smad signaling is a potential approach to prevent pulmonary fibrosis. SMAD7 can bind TβRI and inhibit Smad 2/3 phosphorylation blocking TGF-β1/Smad signaling prevent TGF-β1/Smad2/3 signaling (7,8). Transient expression of Smad7 inhibits bleomycin-induced lung fibrosis in mice (9), suggesting that the expression level of Smad7 is critical to prevent TGF-β1-mediated lung fibrogenesis. Smad7 stability is mainly regulated by both acetylation and ubiquitylation (10,11). Transcription co-activator p300, which interacts with Smad7, directs acetylation of two lysines in Smad7. These two lysines are also targets of ubiquitylation. Therefore, their acetylation prevents Smad7 from ubiquitylation and subsequent degradation.
Histone deacetylase (HDAC) inhibition (HDACi) has been shown to suppress lung fibrosis (12-14). Suberoylanilide hydroxamic acid (SAHA, Zolinza®, vorinostat), a FDA-approved HDACi for the treatment of cutaneous T-cell lymphoma (15), has been shown to have antifibrotic effect in both bleomycin-induced pulmonary fibrosis and chlorhexidine gluconate-induced peritoneal fibrosis in mice (16-19). However, the exact molecular mechanism is unclear. HDAC1 is also involved in TGF-β1-induced EMT in hepatic fibrosis (20). Moreover, inhibition of HDAC1 activity increases the acetylation of Smad7 in 293T cells (11) and expression of HDAC1 was negatively associated with the expression of Smad7 in induced aristolochic acid-renal fibrosis (21). Therefore, we hypothesized that SAHA blocks pulmonary fibrogenesis by stabilizing Smad7 expression level through inhibiting HADC1 activity. To test our hypothesis, we employed a well-accepted paraquat (PQ)–induced lung fibrosis rat model. Here, we showed that SAHA treatment significantly suppressed PQ-induced lung fibrosis, maintained Smad7 expression and reduced Smad3 activity. Our in vitro studies revealed that SAHA exerted its antifibrotic effect through preventing Smad7 from deacetylation most probably by inhibiting TGF-β1-induced HDAC1 activity, and thus decreasing Smad3 activity. Our results suggest that SAHA is a potential antifibrotic reagent for treating PQ-induced lung fibrosis.
Methods
Experimental animals
Male Sprague-Dawley rats (219.2±13.3 g) were obtained from Laboratory Animals Center of Third Military Medical University, China [animal use permit No.: SCXK (yu) 2012-0005]. The study was approved by the Animal Ethics Committee of GuiZhou Medical University (NO. 1203109). The investigation fellows to the National Health and Medical Research Council of China’s Code for the Care and Use of Animals for Scientific Purpose.
In vivo experiment
PQ and SAHA were purchased from Nanjing Red Sun Co., Ltd. and Sigma Aldrich, St. Louis, MO, respectively. PQ were dissolved in a 0.9% saline solution, and SAHA were dissolved in Hydroxypropyl-β-Cyclodextrin (HP-β-CD) solution (22). All animals were housed in a stable environment maintained at 20± 2 °C with a 12-hour light-dark cycle commencing at 6 a.m. A total of 36 male rats were randomly and evenly divided into 3 groups: saline group, PQ group, and PQ + SAHA group. The rats were treated with saline or PQ solution (15 mg/kg) (23,24) by a single intraperitoneal injection. SAHA-HP-β-CD solution were given at 15 mg/kg body weight (25) by gastric gavage every day after the PQ injection, rats in another two group were treated with an equal volume of HP-β-CD solution by gastric gavage from day 2 to 28. On day 28 after PQ injection, rats were sacrificed. Lungs were collected and weighed, with a part of each lung being fixed in 4% paraformaldehyde; the rest of each lung was snap-frozen in liquid nitrogen frozen and then stored at −80 °C for subsequent use.
Histological examination and masson’s trichrome staining
The lungs were fixed in paraformaldehyde and embedded in paraffin. The paraffin-embedded tissues were cut into 5-µm sections with a microtome. The sections were subjected to hematoxylin and eosin (H&E) staining to evaluate histopathological changes. Masson’s trichrome staining was performed to indentify the density of the accumulated collagen fibers.
Immunohistochemical staining
5-µm sections were incubated for 2 h at 60 °C in an oven to remove the paraffin, rehydrated through graded ethanol. Then, the antigen was retrieved by boiling the slides in 10 mM sodium citrate solution (pH 6.0) and non-specificity was blocked with 5% bovine serum albumin for 30 min at room temperature (RT). The sections were then incubated with anti-α-smooth muscle actin (α-SMA, 1:100, Santa Cruz, sc-53142) and anti-Collagen I (Col-I, 1:100, Santa Cruz, sc-59772) antibodies overnight (18 h) at 4 °C. The following day, after rinsing with PBS, the sections were incubated with appropriate secondary antibodies (peroxidase-conjugated goat anti-mouse IgG antibody, ZSGB-Bio, Beijing, China). Subsequently, the sections were stained with 3,3-diaminobenzidine tetrahydrochoride (DAB) for 1 min to visualize the localization of peroxidase conjugates. Finally, the sections were counterstained in hematoxylin.
Hydroxyproline assay
Lung collagen deposition was estimated by measuring the hydroxyproline (HYP) content of lung homogenates with a hydroxyproline assay kit (Nanjing Jiancheng Bioengineering Company, China) in accordance with the manufacturer’s protocol. The absorbance of each sample at 550 nm wavelength was read by a microplate reader. The results were expressed in µg HYP per g wet lung.
Cell culture
Human pulmonary fibroblast (HFL1) cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were grown in a 5% CO2 atmosphere at 37 °C in 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) and Dulbecco’s modified Eagle medium (GIBCO Invitrogen, China), with 1% penicillin/streptomycin. Cells were seeded at 5×105/well in 6-well plates. When cells were near 80% confluence, the culture medium was changed to 1% FBS medium and left overnight. Then, the cells were treated with DMSO only (control group) or 5 µM SAHA (16), with or without recombinant human TGF-β1 (5 ng/mL; Peprotech, USA, 100-21) for 24 or 48 h. Each experiment was repeated at least three times.
Immunofluorescence staining
The cells were grown on coverslips in 6-well plates. Once the cells reached near 70% confluence, the cells were rinsed twice with cold 1xPBS, fixed in a methanol: acetone (1:1) solution at –20 °C for 20 min. Then, the cells were washed with PBS. After blocking with 20% bovine serum albumin diluted in PBS for 30 min, the cells were incubated with anti-Collagen I antibodies (1:100, Santa Cruz, sc-59772) overnight at 4 °C. The following day, cells were rinsed with PBS three times and then incubated with goat-anti-mouse IgG-FITC antibody (1:100, Beyotime, Jiangsu, China) for 60 min at 37 °C in the dark. Finally, the cells were incubated with 4', 6-diamidino-2-phenylindole (DAPI) (Beyotime, Jiangsu, China) as a nuclear counterstain for 15 min at 37 °C in the dark. The cells were then viewed with a LEICA fluorescence microscope in the dark.
Immunoprecipitation and western blotting
Lung homogenates and cell culture samples were sonicated and resuspended in RIPA lysis buffer (Beyotime, Jiangsu, China), supplemented with PMSF and Phosphatase inhibitor cocktail (both from KangChen, Shanghai, China). After centrifugation (12,000 rpm for 20 min at 4 °C), the supernatant from the lysate was collected and the protein concentrations were determined using BCA assay kit. Smad7 was immunoprecipitated from cell lysates using Immunoprecipitation Kit Dynabeads® Protein G (Novex, Life Technologies AS, Norway, 10007D) and the mouse anti-Smad7 antibody (Santa Cruz, sc-365846). Acetylation of Smad7 was assessed by Western blotting using a rabbit anti-acetyl-lysine antibody (1:1000, Immunechem, Burnaby BC, CA, 112206, USA). Protein samples were then separated by 10%SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 5% skim milk in TBS-T for 1 h at room temperature and incubated with anti-β-actin (1:400, Santa Cruz, sc-47778), anti-α-SMA (1:400, Santa Cruz, sc-53142), anti-TGF-β1 (1:400, Santa Cruz, sc-52893), anti-Collagen-I (1:400, Santa Cruz, sc-59772), anti-Smad3 (1:1000, Cell Signaling Technology, 9513), anti-Phospho-Smad3 (1:1000, Cell Signaling Technology, 9520) over night at 4 °C. The next day, after washing with TBS-T 3 times, membranes were incubated with HRP-linked antibody (1:10000, anti-rabbit or anti-mouse IgG, CST) for 1 h at room temperature. At last, bound antibody was detected by ECL Plus (Amersham, Little Chalfont, UK) and captured by Fujifilm model LAS-3000 (Fujifilm Corporation, Australia). Quantification of the Western blot data was performed by measuring the intensity of the hybridization signals using a Quantity one 4.6 Image analysis program. Protein expression levels were calculated after normalizing with β-actin.
HDAC1 activity assays
HDAC1 activity was measured using a HDAC1 colorimetric activity assay kit (GENMED SCIENTIFICS INC., USA, GMS50082.2.2 VA) following the manufacturer’s protocol. In brief, nuclear extracts of HFL1 cells were incubated with Color de Lys substrate at 37 °C for 30–60 min. After incubation, Color de Lys developer was added to the samples and incubated at 37 °C for 15 min. A standard curve was performed according to the manufacturer’s protocol, and absorbance of HDAC1 was measured on the basis of spectrophotometric measures at 405 nm. HDAC1 activity was calculated using the formula from the manufacturer’s protocol.
Statistical analyses
All statistical analyses were performed using SPSS 13.0 software. All Data were shown as mean ± standard deviation (SD). Comparison among groups was made using one-way ANOVA followed by the Student-Newman-Keuls test. A P<0.05 was defined as significant.
Results
SAHA blocked paraquat-induced pulmonary fibrosis
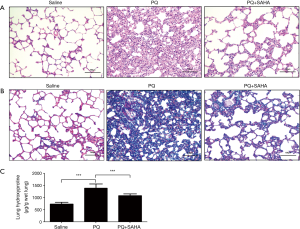
To determine whether SAHA ameliorated pulmonary fibrosis in vivo, we treated rats with PQ-induced lung fibrosis using SAHA, we first observed the effect of SAHA on PQ-induced histological changes using H&E staining and Masson Trichrome staining. H&E staining showed that alveolar septa were thickened with many interstitial cells in PQ group when compared to saline group, while SAHA treatment reduced the extent of alveolitis and the number of interstitial cells (Figure 1A). Masson Trichrome staining study further revealed that the collagen accumulation and the number of interstitial cells were increased in PQ-group compared to saline group (Figure 1B), whereas SAHA treatment significantly reduced these changes by PQ. Hydroxyproline content is a marker of collagen, therefore we assayed hydroxyproline levels in lung tissues. Consistent with our Masson Trichrome staining result, PQ greatly induced hydroxyproline level when compared to saline group, whileas SAHA treatment significantly downregulated increased hydroxyproline by PQ (Figure 1C). Taken together, our results indicated that SAHA attenuated PQ-induced lung fibrogenesis.
SAHA inhibited PQ-induced TGF-β1/Smad signaling
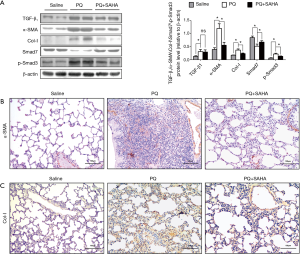
TGF-β1 mRNA levels were increased in paraquat-induced pulmonary fibrosis in rats (26), therefore we investigated whether the TGF-β1/Smad signaling was involved in the antifibrotic effect of SAHA in PQ-induced lung fibrosis. PQ exposure greatly increased the expression of TGF-β1, Smad3 and Collagen-I, and decreased Smad7 expression in lung tissues (Figure 2A), indicating that PQ treatment activated TGF-β1/Smad signaling in lungs. SAHA treatment significantly rescued the loss of Smad7 induced by PQ (Figure 2A). Consistent with increased Smad7 stabilization by SAHA, Smad3 activity and subsequent collage I expression were inhibited by SAHA (Figure 2A). Moreover, TGF-β1 could induced fibroblast transdifferentiation into mycro fibroblasts. Mycro fibroblasts are primary producers of α-SMA and collagen. Immunohistochemical (IHC) staining showed that SAHA treatment remarkably reduced increased α-SMA positive cells (Figure 2B) and collagen deposition (Figure 2C) in the lungs compared to PQ-induced group. These results indicated that SAHA block fibroblast transdifferentiation into mycro fibroblasts. Taken together, these results indicate that SAHA block PQ-induced Smad signaling in lung fibrogenesis.
SAHA prevented TGF-β1-Induced Smad7 from deacetylation
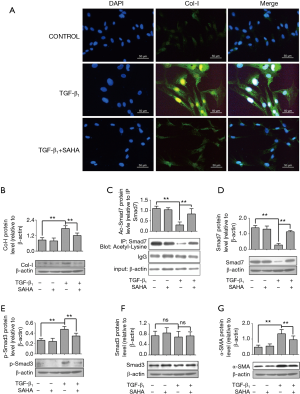
To further determine the molecular mechanism how SAHA influenced Smad7 expression, we used a TGF-β1-induced cellular model. We first confirmed whether SAHA may block TGF-β1–induced fibroblast transdifferentiation into myofibroblasts. HFL1 cells were pretreated with TGF-β1 (5 ng/mL) and then with or without SAHA (5 µM), stained with collagen I. As shown in Figure 3A, TGF-β1 promoted collagen I formation, while SAHA blocked TGF-β1-induced collagen deposition (Figure 3B), suggesting that SAHA suppress fibroblast transdifferentiation. Next, we determined whether Smad7 stabilization by SAHA was associated with its acetylation level using immunoprecipitation assay. TGF-β1 treatment significantly decreased acetyl-Smad7 level, while SAHA treatment remarkably rescued decreased Smad7 acetylation level by TGF-β1 (Figure 3C). We further examined whether rescued Smad7 acetylation by SAHA restored loss of Smad7 expression by TGF-β1. Western Blot analysis showed that SAHA treatment significantly restored Smad7 expression (Figure 3D). Moreover, SAHA treatment inhibited expression levels of phospho-Smad3, α-SMA and collagen I induced by TGF-β1 (Figure 3 E-G).
SAHA suppressed TGF-β1-induced HADAC1 activity in vitro
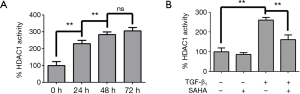
SAHA has been shown to inhibit the deacetylase activity of HDAC1, 2, 3, 6, 8 (27). HDAC1 was confirmed that it’s the strongest deacetylase to deacetylate Smad7 (11). Therefore we wondered if SAHA stabilized Smad7 acetylation by inhibiting HDAC1 activity induced by TGF-β1. Therefore, we examined whether SAHA prevented Smad7 from deacetylation by inhibiting HDAC1 activity. We first measured HDAC1 activity in TGF-β1-stimulated HFL1 cells. TGF-β1 treatment significantly increased HDAC1 activity (Figure 4A) in a time-dependent manner. HDAC1 activity was significantly higher at 48 h than 24 h and HFL1 cells kept in better shape than in 72 h. As shown in Figure 4B, SAHA treatment effectively inhibit TGF-β1-induced HDAC1 activity.
Collectively, these data demonstrated that SAHA suppressed pulmonary fibrosis and fibrogenic signals most probably through inhibiting HDAC1 activity, thus preventing Smad7 from deacetylation.
Discussion
PQ-induced pulmonary fibrosis is very common but lacks effective treatment options. SAHA is shown to be antifibrotic, but the mechanism is not well understood. In this study, we used PQ-induced lung fibrosis rat model and TGF-β1-treated HFL cellular model to investigate the molecular mechanism by which SAHA attenuates lung fibrosis. Our results showed that SAHA greatly inhibited PQ-induced lung fibrosis in rats by restoring Smad7 expression level and decreasing Smad3 activity. Since SAHA inhibits the activities of HDAC 1, 2, 3, 6, and 8, but not SIRT1, which regulates Smad3 deacetylation (28), so SAHA can not affect Smad3 acetylation and deacetylation. Therefore, SAHA exerted its antifibrotic effect through preventing Smad7 from deacetylation, resulting in blocking TGF-β1-mediated lung fibrogenesis.
Our H&E staining and Masson Trichrome staining showed that SAHA treatment lessened thickened alveolar septa induced by PQ and reduced increased numbers of interstitial cells, restored alveolar sizes to normal. IHC staining revealed that SAHA suppressed increased expression of α-SMA and collagen I induced by PQ in rat lung tissues. These results were consistent with antifibrotic effect of SAHA in bleomycin-induced mouse pulmonary fibrosis (18).
Consistent with recent studies that TGF-β1/Smad signaling mediates PQ-induced pulmonary fibrosis in mice, we showed that TGF-β1/Smad signaling was upregulated in PQ-induced rat pulmonary fibrosis, SAHA stabilized Smad7 expression and inhibited Smad3 activity. Immunoprecipitation assay demonstrated that SAHA prevented Smad7 from deacetylation induced by TGF-β1, thus stabilized its expression, inhibited Smad3 activity and subsequently reduced α-SMA and collagen I expression. Our further study demonstrated that SAHA inhibit TGF-β1-induced HDAC1 activity which is critical for Smad7 deacetylation, in agreement with that blocking HDAC1 activity stabilized Smad7 expression in 293T cells (11).
SAHA (Vorinostat) is a FDA-approved drug for T-cell lymphoma treatment and no obvious toxicity in normal human fibroblasts (16) and in healthy mice (13), we evaluated the efficacy of SAHA to attenuate pulmonary fibrosis in a rat model. Our study also observed no obvious side effects of SAHA treatment in the rats. Previous studies report SAHA treatment inhibit collagen 3A formation, whereas we showed the first evidence that SAHA inhibited collagen I deposition, indicating that SAHA inhibits the expression of several collagen types, suggesting that SAHA is a very promising antifibrotic reagent. The limitations in this study include: (I) SAHA is a broad HADC inhibitor, there may be far more targets and more complicated mechanisms than we had in this study; (II) we did not evaluate antifibrotic progress of SAHA and its dose at early days and examine rat lung functions after SAHA administration; (III) we did not verify whether increased Smad7 acetylation by SAHA decreases the level of Smad7 ubiquitylation by PQ. In future experiments, we will further investigate underlying mechanism by which SAHA stabilizes Smad7 expression.
In summary, the present study revealed that inhibiting Smad7 deacetylation by SAHA maintained its stability and blocked TGF-β1/Smad signaling, thus inhibiting fibroblast differentiation into mycro fibroblast in vitro and lung fibrosis in vivo. We, therefore, propose that blockade of Smad7 deacetylation may prove an effective therapeutic strategy to inhibit the progression of pulmonary fibrosis.
Acknowledgements
Funding: This work was supported in part by the National Natural Sciences Foundation of China (No. 81360116) and the Science Technology Office Foundation of Guizhou province [Qian SY (2013) 3175].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Animal Ethics Committee of Guizhou Medical University (No. 1203109) and written informed consent was obtained from all patients.
References
- Vadivelan M, Chellappan A, Suryanarayana BS. The 'golden hour' in paraquat poisoning. Toxicol Int 2014;21:339-40. [Crossref] [PubMed]
- Yamashita M, Yamashita M, Ando Y. A long-term follow-up of lung function in survivors of paraquat poisoning. Hum Exp Toxicol 2000;19:99-103. [Crossref] [PubMed]
- Yao R, Cao Y, He YR, et al. Adiponectin attenuates lung fibroblasts activation and pulmonary fibrosis induced by paraquat. PLoS One 2015;10:e0125169. [Crossref] [PubMed]
- Cherukuri H. Demographics, clinical characteristics and management of herbicide poisoning in tertiary care hospital. Toxicol Int 2014;21:209-13. [Crossref] [PubMed]
- Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol 2003;29:397-404. [Crossref] [PubMed]
- Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 2006;103:13180-5. [Crossref] [PubMed]
- Nakao A, Afrakhte M, Morén A, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 1997;389:631-5. [Crossref] [PubMed]
- Nakao A, Imamura T, Souchelnytskyi S, et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J 1997;16:5353-62. [Crossref] [PubMed]
- Nakao A, Fujii M, Matsumura R, et al. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest 1999;104:5-11. [Crossref] [PubMed]
- Grönroos E, Hellman U, Heldin CH, et al. Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell 2002;10:483-93. [Crossref] [PubMed]
- Simonsson M, Heldin CH, Ericsson J, et al. The balance between acetylation and deacetylation controls Smad7 stability. J Biol Chem 2005;280:21797-803. [Crossref] [PubMed]
- Davies ER, Haitchi HM, Thatcher TH, et al. Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis. Am J Respir Cell Mol Biol 2012;46:687-94. [Crossref] [PubMed]
- Sanders YY, Hagood JS, Liu H, et al. Histone deacetylase inhibition promotes fibroblast apoptosis and ameliorates pulmonary fibrosis in mice. Eur Respir J 2014;43:1448-58. [Crossref] [PubMed]
- Cetinkaya M, Cansev M, Cekmez F, et al. Protective Effects of Valproic Acid, a Histone Deacetylase Inhibitor, against Hyperoxic Lung Injury in a Neonatal Rat Model. PLoS One 2015;10:e0126028. [Crossref] [PubMed]
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 2007;25:84-90. [Crossref] [PubMed]
- Wang Z, Chen C, Finger SN, et al. Suberoylanilide hydroxamic acid: a potential epigenetic therapeutic agent for lung fibrosis? Eur Respir J 2009;34:145-55. [Crossref] [PubMed]
- Guo W, Shan B, Klingsberg RC, et al. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol 2009;297:L864-70. [Crossref] [PubMed]
- Zhang X, Liu H, Hock T, et al. Histone deacetylase inhibition downregulates collagen 3A1 in fibrotic lung fibroblasts. Int J Mol Sci 2013;14:19605-17. [Crossref] [PubMed]
- Io K, Nishino T, Obata Y, et al. SAHA Suppresses Peritoneal Fibrosis in Mice. Perit Dial Int 2015;35:246-58. [Crossref] [PubMed]
- Lei W, Zhang K, Pan X, et al. Histone deacetylase 1 is required for transforming growth factor-beta1-induced epithelial-mesenchymal transition. Int J Biochem Cell Biol 2010;42:1489-97. [Crossref] [PubMed]
- Tian Y, Yang Y, Gao L, et al. Expression of histone deacetylase-1 and p300 in aristolochic acid nephropathy models. Toxicol Mech Methods 2014;24:377-84. [Crossref] [PubMed]
- Hockly E, Richon VM, Woodman B, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc Natl Acad Sci U S A 2003;100:2041-6. [Crossref] [PubMed]
- Cegla UH, Kroidl RF, Kronberger H, et al. Experimental model of pulmonary fibrosis in the rat using paraquat injection. Pneumonologie 1975;152:65-74. [Article in German]. [Crossref] [PubMed]
- Seifirad S, Keshavarz A, Taslimi S, et al. Effect of pirfenidone on pulmonary fibrosis due to paraquat poisoning in rats. Clin Toxicol (Phila) 2012;50:754-8. [Crossref] [PubMed]
- Cohen LA, Amin S, Marks PA, et al. Chemoprevention of carcinogen-induced mammary tumorigenesis by the hybrid polar cytodifferentiation agent, suberanilohydroxamic acid (SAHA). Anticancer Res 1999;19:4999-5005. [PubMed]
- Chen J, Zeng T, Zhao X, et al. Docosahexaenoic acid (DHA) ameliorates paraquat-induced pulmonary fibrosis in rats possibly through up-regulation of Smad 7 and SnoN. Food Chem Toxicol 2013;57:330-7. [Crossref] [PubMed]
- Cavasin MA, Stenmark KR, McKinsey TA. Emerging roles for histone deacetylases in pulmonary hypertension and right ventricular remodeling (2013 Grover Conference series). Pulm Circ 2015;5:63-72. [Crossref] [PubMed]
- Li J, Qu X, Ricardo SD, et al. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 2010;177:1065-71. [Crossref] [PubMed]


