SBRT versus lobectomy in stage I NSCLC: knowns, unknowns and its interpretation
Stereotactic body radiotherapy (SBRT) has become the guideline recommended treatment for patients with stage I non-small cell lung cancer (NSCLC), who are medically inoperable due to their comorbidities (1). In historical comparisons, SBRT has achieved substantially improved local tumor control compared to conventional radiotherapy, which has translated into improved overall survival (OS) (2). Additionally, SBRT is safe and effective even in highly fragile patient populations suffering from serve comorbidities or being at very advanced age.
The promising results of SBRT in medically inoperable patients have prompted the evaluation of SBRT in healthy patients, where lobectomy and systematic hilar and mediastinal lymph node dissection is the standard of care. Ideally, randomized controlled trials would have answered the question about the value of SBRT compared to surgical lobectomy. Three randomized controlled trials comparing SBRT and lobectomy have been started (ROSEL; STARS; RTOG 1021/ACOSOG Z4099) but closed early due to very poor patient accrual. Further trials comparing SBRT with surgical resection are planned or are already open (SABRTooth; STABLE-MATES; VALOR) and more advanced techniques of randomization and patient information will be used in these trials. However, we will remain without highest level of evidence for several years.
Knowns
Several sources of evidence are today available to guide the current discussion of SBRT compared to lobectomy. All are below the evidence level of randomized controlled trials, all have their specific methodological limitations and interpretation of all these studies needs to consider these limitations.
Data of two randomized trials (ROSEL & STARS) have been pooled and a joint analysis has been performed (3). Only 58 patients in total were available for analysis, which is a major limitation of this study. Median age of the patients was 67 years without differences between the two cohorts. After sufficient follow-up of >40 months, 3 years OS was 95% and 79% in the SBRT and surgical group (P=0.037), respectively. No differences in local recurrence and recurrence free survival were observed. Grade 3 & 4 complications were observed in 44% of the patients after lobectomy whereas only 10% of the patients developed grade 3 toxicity after SBRT. Only 22 patients were randomized in the ROSEL trial and an exploratory analysis described patient reported outcome. Overall, quality of life was consistently better after SBRT compared to lobectomy but reached statistical significance only in global health status (4).
Only one prospective single-arm phase II trial using SBRT in medical operable patients has been fully reported, yet. The JCOG0403 study included patients with cT1cN0 NSCLC and was open for medically operable and medically inoperable patients (5): the status operable was defined as an expected postoperative forced expiratory volume 1.0 >800 mL; PaO2 >65 torr (under room air) and no severe cardiac morbidity and no severe diabetes mellitus. Sixty four of 164 eligible patients were medically operable and their median age was 79 years. After a median follow-up of 67 months, 3- and 5-year OS was 76.5% and 54%, respectively. Only 6.2% of the patients developed grade 3 toxicity, no grade 4 toxicity was observed. Results of the RTOG 0618 study were reported at the ASCO 2013 conference and 2-year OS of 84.4% was reported in 26 patients; full publication of this study is eagerly awaited.
To the best of our knowledge, this is all prospective data about stage I NSCLC patients, who were treated with SBRT despite being medically operable. However, several retrospective single-center and multi-center studies reported their experiences of SBRT in medically operable patients, who refused surgical resection (Table 1). Criteria and methodology of defining operable vs. inoperable varied between studies. Nevertheless, 3 years OS ranged consistently between 79% and 86% (6-10). Komiyama et al. have reported the largest multi-institutional study of 661 medically operable patients treated with SBRT (10). After a median follow-up of 35 months, 3-year OS was 79% and reached 80% for the cohort of patients treated with sufficiently high radiation doses of ≥100 Gy BED. Two studies reported 5-year OS, which ranged between 51.3% and 69.5% (7,9). Despite the lack of randomized trials, there is consequently relevant and important evidence available.

Full table
Unknowns
In contrast to all studies discussed above, the study by Rosen et al. falls into the category of registry and population-based studies, where big and mostly national databases are used for outcome comparisons. Rosen et al. analysed the National Cancer Database, which captures about 70% of all NSCLC cases in the United States. For 1781 so-called operable patients treated with SBRT the 5-year OS was only 29%: operable was defined as having a “Charlson-Deyo comorbidity index of zero”. A subset of patients (n=235) was defined based on the coding of “Surgery of the primary site was not performed; it was recommended by the patient’s physician, but this treatment was refused by the patient, the patient’s family member, or the patient’s guardian”. In this cohort 5-year OS was better but still only 40%. In all comparisons, OS after SBRT was significantly worse compared to lobectomy. Similar analyses have been performed using the SEER database and inconsistent results of SBRT compared to lobectomy have been reported (11-14).
Direct and one-by-one comparison of SBRT and surgical results in such registry studies is not possible and is just like comparing of apples and oranges. The vast majority of the patients treated with SBRT was inoperable and represent a high-risk patient population, which is obviously different to operable patients treated with surgery. Therefore, all these studies have in common that advanced and complex statistics is used to make surgical and SBRT patient cohorts as comparable as possible. However, the majority of the available studies including the one by Rosen et al. have in common that well known prognostic factors are unknown and therefore potential imbalances cannot be corrected. Especially performance status, pulmonary function and detailed comorbidity score are essential for accurate patient characterization and estimation of the risk of non-cancer death (15-17). The value of including these factors into comparative analyses between SBRT and lobectomy is highlighted by the study of Verstegen et al., where propensity matching between patients treated with SBRT and VATS lobectomy was based on the following parameters (18): cTNM stage, age, gender, Charlson comorbidity score, lung function and performance score. The comparison of 64 SBRT and 64 VATS well balanced patients resulted in 3-year OS of 79.6% and 76.9%, respectively. The true value of such registry studies remains unknown.
Interpretation
At this point, I will come back to the population of medically inoperable patients treated with SBRT. Several prospective phase II studies have been conducted and results in terms of OS are highly consistent. Despite the definition of medical inoperability and the risk for non-cancer risk most likely varied between institutions and studies, 3-year OS ranged between 50–60% (Table 2). Furthermore, very similar and consistent OS is also achieved in large retrospective and multi-institutional studies.
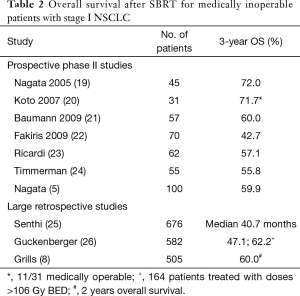
Full table
How do all these data fit into one picture? OS in medically operable patients treated with SBRT is about 80% after 3 years based on prospective and retrospective studies discussed above. In medically inoperable patients, 3-year OS is reduced to about 50–60%; OS varies due to the competing risk of death as a consequence of the underlying comorbidities. In the study by Rosen et al., 3-year OS was about 50–60% in the so-call “operable” patients treated with SBRT; this is very similar to survival observed in inoperable patient cohorts but substantially worse than survival observed in all studies about operable patients. In the lobectomy patients, 3-year OS was about 80%, which fits well into current literature data.
The reason for this “poor” OS after SBRT compared to lobectomy in the study of Rosen et al. remains largely unknown. What is the contribution of a potentially more effective lobectomy compared to SBRT? Surgical lymph node dissection is mostly a diagnostic and not a therapeutic procedure and it’s direct influence on OS is small or even not existing (27). About 12% of the surgical patients in the study of Rosen et al. had pathological positive nodes, of which 70% received adjuvant chemotherapy. However, the small benefit of adjuvant chemotherapy in a small proportion of the patients does also not explain the large difference in OS between SBRT and lobectomy (28). Finally, SBRT achieves local tumor control consistently in minimum 90% of the patients, which does not leave much room for improvements for surgery.
In conclusion, the most plausible explanation for the findings of Rosen et al. is the hypothesis, that the National Cancer Database did not allow for accurate identification of truly operable patients treated with SBRT. Patients treated with SBRT were most likely different to surgical patients in terms of their comorbidities and their consequential risk of non-cancer death, despite all statistical means trying to minimize such bias. These differences in patient characteristics may then explain part if not all observed OS differences between SBRT and lobectomy. Future comparative studies are urgently needed and they need to provide more in-depth information about the patients’ comorbidities and their non-cancer risk factors.
Until such data become available, surgical lobectomy remains the standard of care for appropriately selected patients. However, SBRT should be recognized as an alternative, which offers similar or maybe even identical OS compared to lobectomy. Consequently, patients need to be informed about available alternatives and their specific pros and cons—not only in terms of OS but also quality of life and toxicity—instead of communicating one methodology as the only curative option.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Feichao Bao (Department of Thoracic Surgery, The First Affiliated Hospital, Zhejiang University, Hangzhou, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153-9. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Louie AV, van Werkhoven E, Chen H, et al. Patient reported outcomes following stereotactic ablative radiotherapy or surgery for stage IA non-small-cell lung cancer: Results from the ROSEL multicenter randomized trial. Radiother Oncol 2015;117:44-8. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Uematsu M, Shioda A, Suda A, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys 2001;51:666-70. [Crossref] [PubMed]
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [Crossref] [PubMed]
- Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol 2012;7:1382-93. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Komiyama T, Onishi H, Shioyama Y, et al. Japanese multicenter study of stereotactic body radiotherapy for 661 medically operable patients with stage I non-small cell lung cancer. Journal of Thoracic Oncology 2015;10:S210-S1.
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Yu JB, Soulos PR, Cramer LD, et al. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer 2015;121:2341-9. [Crossref] [PubMed]
- Monirul Islam KM, Shostrom V, Kessinger A, et al. Outcomes following surgical treatment compared to radiation for stage I NSCLC: a SEER database analysis. Lung Cancer 2013;82:90-4. [Crossref] [PubMed]
- Kopek N, Paludan M, Petersen J, et al. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol 2009;93:402-7. [Crossref] [PubMed]
- Guckenberger M, Kestin LL, Hope AJ, et al. Is there a lower limit of pretreatment pulmonary function for safe and effective stereotactic body radiotherapy for early-stage non-small cell lung cancer? J Thorac Oncol 2012;7:542-51. [Crossref] [PubMed]
- Klement RJ, Belderbos J, Grills I, et al. Prediction of early death in patients with early-stage NSCLC-can we select patients without a potential benefit of SBRT as a curative treatment approach? J Thorac Oncol 2016;11:1132-9. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427-31. [Crossref] [PubMed]
- Koto M, Takai Y, Ogawa Y, et al. A phase II study on stereotactic body radiotherapy for stage I non-small cell lung cancer. Radiother Oncol 2007;85:429-34. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677-82. [Crossref] [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer 2010;68:72-7. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- Guckenberger M, Allgäuer M, Appold S, et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 2013;8:1050-8. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015.CD011430. [PubMed]


