Carinal resection and sleeve pneumonectomy
Introduction
Neoplasms involving the carina and/or distal trachea without systemic or lymphatic metastases are uncommon but not rare (1,2). Most of these patients were diagnosed at an advanced stage, and were likely to be no longer candidates for surgical resection (1). On the other hand, patients with limited involvement of the carina or distal trachea can still be considered for extended surgical resection with airway reconstruction (1).
These lesions constitute a major challenge for a thoracic surgeon in order to achieve a complete resection. The difficulty includes the dissection of the trachea and main bronchi, the resection of the carina and the reconstruction of the trachea and bronchus (3). The major difficulties are “extended resections” where the re-anastomosed structures are under tension and the surgeon has to judge how far he can go with his resection margins (4) Furthermore there are different variations for reconstruction and since the procedures are rare even in high volume centers only few surgeons have relevant experience. Although the technique is well known, the incidence of high postoperative complication rate makes this type of procedures more challenging not only for the patient but also for the surgeon (4).
Improved patient selection, anesthetic management, surgical technique and better postoperative management reduced the rate of postoperative morbidity and mortality (5).
Indications and contraindications
In 1950, Abbott reported four patients who required right pneumonectomy with en bloc excision of the carina, lateral wall of the trachea and part of the left main bronchus (6). In 1959, Gibbon reported the first case of sleeve pneumonectomy (SP) (7). In 1957, Barclay et al. reported their clinical experience with carina resection and primary reconstruction (8). In early eighties, Grillo published his experience on carina resection in 36 patients and pioneered the modern era of the tracheal surgery (9).
Indications for a SP or a carinal resection (CR) include non-small cell lung cancer, other airway tumors and benign or inflammatory strictures (10).
All patients who are candidates for SP or CR should be subjected to thorough medical screening with special attention to cardiac and pulmonary function and co-existing medical diseases. Complete pulmonary function tests, arterial blood gas analysis and quantitative ventilation/perfusion scans should be performed to help in the assessment of pre-and postoperative lung function (11,12).
The preoperative staging includes chest X-ray, computed tomography (CT) scan of the chest and upper abdomen, positron emission tomography (PET) and bronchoscopy. Bronchoscopy is the most important tool as it usually helps mostly to identify a possible candidate for SP or CR. The degree of invasion should be documented by biopsies and to take additional random biopsies 1 or 2 cm above the visible tumor is recommended (5). These biopsies are important in order to achieve a complete resection as a tension free anastomosis is possible if the tumor does not extend beyond 2 cm of the lower trachea or beyond 1.5 cm of the opposite main bronchus (5). For a tension free reconstruction a 4 cm distance between lower trachea and opposite bronchus is advocated (13).
Preoperative irradiation more than 45 cGy is a relative contraindication to SP and even has been considered an absolute contraindication by some (5).
Preoperative mediastinoscopy is indicated in all patients who are candidates for a SP or CR since patients with lymph node metastases in the upper mediastinum have generally a poor prognosis (3,5,12). Some centers perform preoperative mediastinoscopy in PET scan positive patients (12). Patients with histologically proven N2 disease undergo induction chemotherapy and then re-assessed with a repeat PET scan: responders or those with stable disease are scheduled for surgery. Repeat mediastinoscopy is not recommended in order to avoid excessive devascularization of the trachea (12). It has also been reported that the presence of metastatic mediastinal nodes to be a potential contraindication for surgery (14). These authors recommend performing mediastinoscopy routinely at the time of the planned CR to avoid the development of scar tissue along the trachea (14). On the other hand, widespread use of endobronchial ultrasound to stage the mediastinum has been reported to prevent disruption of the surgical planes which might lead to scaring and limitation of airway mobility (15,16).
Special situations that should be kept in mind for carina resection and SP have recently been summarized in a review by Tapias et al. (16). They mentioned that as chronic use of steroids might lead to impairment of anastomotic healing, the patients should be weaned from the steroids at least 2 to 4 weeks before surgery, Secondly, as positive pressure ventilation can apply stress on the airway anastomosis, the need for postoperative mechanical ventilation might lead to increased morbidity following surgery. Thirdly, patients who received neoadjuvant therapy, especially radiotherapy, are under risk for anastomotic complications due to tissue ischemia. These authors recommend coverage of anastomosis with a vascularized tissue (16).
Anesthesia and surgical technique
During the operation a close cooperation of surgeon and anesthesiologist is required. Anesthetic techniques aim at maintaining adequate anesthesia and gas exchange while providing a good surgical exposure (5). Generally anesthesia is maintained by intubation of the remaining bronchus using a sterile endotracheal tube connected to sterile tubing passed off the anesthesiologist (cross-field ventilation). In between intermittent apnea can be used to allow precise placement of the anastomotic sutures. As the far edge and posterior wall of the anastomosis is completed the original endotracheal tube is advanced into the bronchus. Then anterior wall of the anastomosis can be reconstructed. High frequency jet ventilation (HFJV) is another option. Since the tube used for ventilation is of small diameter, the surgical reconstruction is much easier to perform in comparison to a regular endobronchial tube. The use of extracorporeal membrane oxygenation (ECMO) to perform such complex operations has also been reported recently with good results (17,18). It is restricted to situation where single lung ventilation is not maintaining sufficient gas exchange
Right SP
Although most of the surgeons prefer posterolateral thoracotomy the current authors prefer an anterolateral thoracotomy. The technical details from the authors’ have been published recently (4). If the tumor is found to be resectable during the operation, the pulmonary artery and veins are encircled (if necessary intrapericardial) and divided with a vascular stapler. The azygos vein is divided with a stapler and the vena cava superior is mobilized in order to obtain a better exposure. The distal trachea and the left main bronchus are exposed, mobilized, and encircled with tapes. It is important not to mobilize distal trachea too extensive in order to preserve maximal blood supply. The trachea is transected above the carina and the left main bronchus near its origin (2). The distance of the resection line to the tumor should preferentially be 1 cm (Figure 1A). Then the traction sutures are placed at the edge between the cartilaginous and membranous parts. At this moment anesthesia is maintained either with cross-field ventilation or via HFJV (Figure 1B).
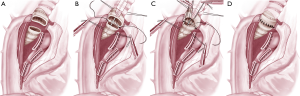
Although there are several ways to reconstruct the airway continuity, the authors prefer the technique where running sutures are used for membranous part (Figure 1B) and interrupted sutures for the cartilaginous part (double armed PDS 4/0, RB1 needle). First the stay suture at the contralateral edge is tied and the membranous part is sutured in a running fashion beginning from the far edge. After completing the membranous part the stay suture on the near edge is tied. For the reconstruction of the cartilaginous part we start from the far edge with already knotted suture of this edge with 2 or 3 continuous stiches and then insert interrupted sutures (Figure 1C). The sutures are tied after they all have been inserted (Figure 1D). Before tying the sutures cross-field ventilation is replaced with the original endotracheal tube. The anastomosis is covered with a viable tissue either with intercostal muscle flap or pericardial fat (5). Then the anastomosis checked for air leaks under water following flexible bronchoscopy.
Left SP
Left SP is rarely performed. The reason for that is the left main bronchus is longer than the right main bronchus and the left main bronchus usually invades the structures of the subaortic space (19). A positive resection margin after a standard left pneumonectomy is the most common indication for left SP.
For a planned left SP there are different approaches. It can be performed via median sternotomy (5), hemi-clamshell incision or as suggested by Porhanov et al. transection of the pulmonary artery and veins with video-assisted thoracoscopic surgery (VATS) and completion the procedure via sternotomy (3).
For an unplanned left SP (for example, positive resection margin) SP can be performed at the same operation (one-stage procedure) (13,20,21) or later (two-stage procedure). For two-stage procedure, the pneumonectomy is completed accepting a positive resection margin and thereafter SP is done from the right side or through a sternotomy (5).
Carina resection without pulmonary resection
The most common approach for CR is right thoracotomy through the fourth or fifth intercostal space. Left side approach due to left aortic arch is difficult or impossible from our view (11). Bilateral sub-mammary trans-sternal “clamshell” thoracotomy and median sternotomy are other ways to approach carina in selected patients (22). Video-assisted thoracoscopic tracheal resection and carinal reconstruction has also been reported (23).
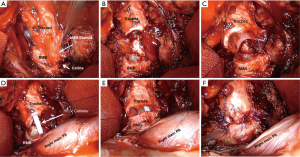
Various techniques for reconstruction following CR have been proposed. They all depend in the extent of the resected trachea, left and right main bronchus (11). Carina resection can also be performed due to bronchopleural fistula following left or right pneumonectomy (Figure 2).
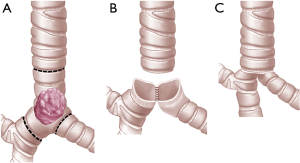
For limited resections of the carina, left and right main bronchus can be re-approximated to form a “neo-carina” (10,11). For CR with neo-carina formation the dissection of the lower trachea and the contralateral main bronchus should not be too extensive in order to preserve local blood supply as much as possible. First we reconstruct the neo-carina. For this we insert the first stich between the cartilaginous and membranous portion of the two main bronchi. After knotting the cartilaginous part which is in contact with each other is approximated in a running suture (neo-carina). Then the membranous part of the trachea is reconstructed with the membranous part of the both bronchi with a running suture beginning from the far edge and coming to the near edge. The anterior wall (cartilaginous portion) is completed with interrupted sutures placed and tied at the end (Figures 3,4). For CR with extensive airway resection the trachea can be anastomosed end-to-end with either right or left main bronchus, with the contralateral bronchus re-implanted into the side of the trachea (24).
Postoperative care
The patients are generally extubated following the procedure in the operating room. Before extubation flexible bronchoscopy should be done in order to check the anastomosis again and clear the endobronchial secretions. The postoperative bronchorrhea should be preferentially treated with chest physiotherapy. A bedside flexible bronchoscopy is rarely needed. In severe cases prophylactic mini-tracheotomy is advocated (5,25).
Postoperative pain control is performed with epidural anesthesia and with paracetamol and metamizole. Antibiotic prophylaxis is performed with amoxicillin clavulanic acid at least 7 days.
Following CR and reconstruction, a heavy stitch between the chin and chest was recommended avoiding excessive tension in the early postoperative period for 7 days, however we do not use this anymore since 15 years without any complication at the airways (11).
Morbidity, mortality and survival
After a carina resection, in spite of the careful patient selection and meticulous surgical technique, complications may occur in the postoperative period. The mortality rate following carina resection ranges between 3% to 20% with an overall morbidity rate of 11% to 50% (3,14,16,24,26-29). Acute respiratory distress syndrome (ARDS) is a life-threatening complication which occurs in up to 20% of SP and has a mortality rate of 50% to 100% (12,14,27). Ventilator-induced injury and fluid overload during surgery are referred as the risk factors (27,30,31). Porhanov et al reported the use of HFJV as a risk factor for development of ARDS which occurred in 8 of 11 cases (3). Contrary to this observation, Rea et al. reported only 2% ARDS in those managed with HFJV (12).
Anastomotic complications vary from granulation tissue, necrosis, mucosal sloughing, micro-fistula to life-threatening dehiscence of the anastomosis (12). According to Deslauriers et al. experience bronchopleural fistulas (BPF) are uncommon if the anastomosis is healthy and covered with a viable tissue (5). High dose preoperative radiotherapy can increase the risk of BPF formation (28). Jensik et al. reported 6 BPF in their 34 patients (32). Five of those had preoperative radiotherapy. Contrary to this, Gonfiotti et al. do not find preoperative chemoradiotherapy as a risk factor for airway anastomotic complications (33). Airway resection limited to 4 cm, avoidance of bronchial devascularization, precise anastomotic suture technique and careful handling of tissues are key factors in preventing anastomotic problems (5).
The role of induction therapy on morbidity and mortality is controversial. de Perrot et al. reported an increase in mortality after SP from 6.7% to 13% (14). On the other hand, Macchiarini et al. reported 2% mortality following SP following preoperative chemoradiation (34).
The reported mortality rates following SP ranges from 2% to 20% (Table 1). In the series reported after 2005 the mortality rate ranges from 2% to 8.5% (3,12,14,27-29,34-36).
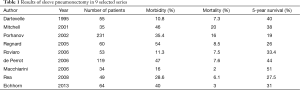
Full table
Acknowledgements
The authors thank Carol De-Simio-Hilton for drawings of the figures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Shin S, Park JS, Shim YM, et al. Carinal resection and reconstruction in thoracic malignancies. J Surg Oncol 2014;110:239-44. [Crossref] [PubMed]
- Perelman MI, Koroleva NS. Primary tumors of the trachea. In: Grillo HC, Eschapasse H. editors. Major challenges. Philadelphia, PA: Saunders, 1987:91-106.
- Porhanov VA, Poliakov IS, Selvaschuk AP, et al. Indications and results of sleeve carinal resection. Eur J Cardiothorac Surg 2002;22:685-94. [Crossref] [PubMed]
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. Thorac Surg Clin 2014;24:77-83. [Crossref] [PubMed]
- Deslauriers J, Grégoire J, Jacques LF, et al. Sleeve pneumonectomy. Thorac Surg Clin 2004;14:183-90. [Crossref] [PubMed]
- Abbott OA. Experiences with the surgical resection of the human carina, tracheal wall, and contralateral bronchial wall in cases of right total pneumonectomy. J Thorac Surg 1950;19:906-22. [PubMed]
- Chamberlain JM, Mc Neill TM, Parnassa P, et al. Bronchogenic carcinoma. An aggressive surgical attitude. J Thorac Cardiovasc Surg 1959;38:727-45. [PubMed]
- Barclay RS, McSwan N, Welsh TM. Tracheal reconstruction without the use of grafts. Thorax 1957;12:177-80. [Crossref] [PubMed]
- Grillo HC. Carinal reconstruction. Ann Thorac Surg 1982;34:356-73. [Crossref] [PubMed]
- Mitchell JD. Carinal resection and reconstruction. Chest Surg Clin N Am 2003;13:315-29. [Crossref] [PubMed]
- Lanuti M, Mathisen DJ. Carinal resection. Thorac Surg Clin 2004;14:199-209. [Crossref] [PubMed]
- Rea F, Marulli G, Schiavon M, et al. Tracheal sleeve pneumonectomy for non small cell lung cancer (NSCLC): short and long-term results in a single institution. Lung Cancer 2008;61:202-8. [Crossref] [PubMed]
- Dartevelle P, Macchiarini P. Techniques of pneumonectomy. Sleeve pneumonectomy. Chest Surg Clin N Am 1999;9:407-17. xi. [PubMed]
- de Perrot M, Fadel E, Mercier O, et al. Long-term results after carinal resection for carcinoma: does the benefit warrant the risk? J Thorac Cardiovasc Surg 2006;131:81-9. [Crossref] [PubMed]
- Blasberg JD, Wright CD. Surgical considerations in tracheal and carinal resection. Semin Cardiothorac Vasc Anesth 2012;16:190-5. [Crossref] [PubMed]
- Tapias LF, Ott HC, Mathisen DJ. Complications Following Carinal Resections and Sleeve Resections. Thorac Surg Clin 2015;25:435-47. [Crossref] [PubMed]
- Keeyapaj W, Alfirevic A. Carinal resection using an airway exchange catheter-assisted venovenous ECMO technique. Can J Anaesth 2012;59:1075-6. [Crossref] [PubMed]
- Lei J, Su K, Li XF, et al. ECMO-assisted carinal resection and reconstruction after left pneumonectomy. J Cardiothorac Surg 2010;5:89. [Crossref] [PubMed]
- Roviaro G, Varoli F, Romanelli A, et al. Complications of tracheal sleeve pneumonectomy: personal experience and overview of the literature. J Thorac Cardiovasc Surg 2001;121:234-40. [Crossref] [PubMed]
- Grillo HC. Carcinoma of the lung: what can be done if the carina is involved? Am J Surg 1982;143:694-5. [Crossref] [PubMed]
- Smith RA, Nigam BK. Resection of proximal left main bronchus carcinoma. Thorax 1979;34:616-20. [Crossref] [PubMed]
- Pearson FG, Todd TR, Cooper JD. Experience with primary neoplasms of the trachea and carina. J Thorac Cardiovasc Surg 1984;88:511-8. [PubMed]
- He J, Wang W, Li J, et al. Video-assisted thoracoscopic surgery tracheal resection and carinal reconstruction for tracheal adenoid cystic carcinoma. J Thorac Dis 2016;8:198-203. [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52; discussion 52-3. [Crossref] [PubMed]
- Bonde P, Papachristos I, McCraith A, et al. Sputum retention after lung operation: prospective, randomized trial shows superiority of prophylactic minitracheostomy in high-risk patients. Ann Thorac Surg 2002;74:196-202; discussion 202-3. [Crossref] [PubMed]
- Tedder M, Anstadt MP, Tedder SD, et al. Current morbidity, mortality, and survival after bronchoplastic procedures for malignancy. Ann Thorac Surg 1992;54:387-91. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Resection for bronchogenic carcinoma involving the carina: long-term results and effect of nodal status on outcome. J Thorac Cardiovasc Surg 2001;121:465-71. [Crossref] [PubMed]
- Roviaro G, Vergani C, Maciocco M, et al. Tracheal sleeve pneumonectomy: long-term outcome. Lung Cancer 2006;52:105-10. [Crossref] [PubMed]
- Regnard JF, Perrotin C, Giovannetti R, et al. Resection for tumors with carinal involvement: technical aspects, results, and prognostic factors. Ann Thorac Surg 2005;80:1841-6. [Crossref] [PubMed]
- Deslauriers J, Aucoin A, Grégoire J. Postpneumonectomy pulmonary edema. Chest Surg Clin N Am 1998;8:611-31. ix. [PubMed]
- Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558-65. [Crossref] [PubMed]
- Jensik RJ, Faber LP, Kittle CF, et al. Survival in patients undergoing tracheal sleeve pneumonectomy for bronchogenic carcinoma. J Thorac Cardiovasc Surg 1982;84:489-96. [PubMed]
- Gonfiotti A, Jaus MO, Barale D, et al. Carinal resection. Thorac Surg Clin 2014;24:477-84. [Crossref] [PubMed]
- Macchiarini P, Altmayer M, Go T, et al. Technical innovations of carinal resection for nonsmall-cell lung cancer. Ann Thorac Surg 2006;82:1989-97; discussion 1997.
- Dartevelle PG, Macchiarini P, Chapelier AR. 1986: Tracheal sleeve pneumonectomy for bronchogenic carcinoma: report of 55 cases. Updated in 1995. Ann Thorac Surg 1995;60:1854-5. [Crossref] [PubMed]
- Eichhorn F, Storz K, Hoffmann H, et al. Sleeve pneumonectomy for central non-small cell lung cancer: indications, complications, and survival. Ann Thorac Surg 2013;96:253-8. [Crossref] [PubMed]