VATS lobectomy for lung cancer was first described
in the early 1990s (
9-10). The first randomized controlled
trial by Kirby concluded that VATS lobectomy was
associated with lower postoperative complications, but
not with significant decrease in intraoperative blood
loss, duration of chest tube drainage, length of stay, or
postoperative pain (
11). McKenna et al. reported the
largest single-institutional series on VATS lobectomy to
date (
12). In their series of 1,100 patients, the mortality
rate was only 0.8% and morbidity rate was 15.3%. The
mean length of hospital stay was 4.78 days. The shortterm postoperative results suggested that VATS lobectomy
is a safe and feasible surgical procedure in the hands of
experienced surgeons. The Cancer and Leukemia Group
B (CALGB) 39802 prospective (
6), multi-institutional
study elucidated the technical feasibility and safety of
standardized VATS lobectomy for early-stage NSCLC.
It was designed to evaluate success rate, morbidity,
mortality, cancer recurrence, and failure-free survival. The
study demonstrated technical feasibility and showed low
complication and chest tube duration.
Lobectomy remained the standard surgical resection
for early lung cancer. However, with the increasing
prevalence of computed tomography application, early
lung cancer with small size nodule became more easily
detectable. There was a resurgence of interest in anatomic
segmentectomy for very early lung cancer, especially in
patients with compromised cardio-pulmonary function, who might not tolerate lobectomy due to inadequate
postoperative reserved pulmonary function (
13). Growing
data suggested that segmentectomy was an alternative
to lobectomy in patients with clinical T1N0M0 status,
especially when tumor diameter was less than 2 cm. This
anatomic segmental resection could be performed safely
without compromising oncologic results (
13-15). In some
institutions, segmentectomy with radical lymph node
dissection was performed not only in high-risk patients
but also in low-risk patients with clinical T1N0M0 and
tumors ≤2 cm in diameter (
16-17). It could offer the benefit
of significantly better preservation of pulmonary function
compared with lobectomy (
18-19). In our institution,
segmentectomy was designed as an alternative standard
resection for peripheral clinical T1N0M0 lung cancer with
diameter ≦2 cm regardless of the risk level. According to
the published data, we considered segmentectomy could
preserve more pulmonary function without compromising
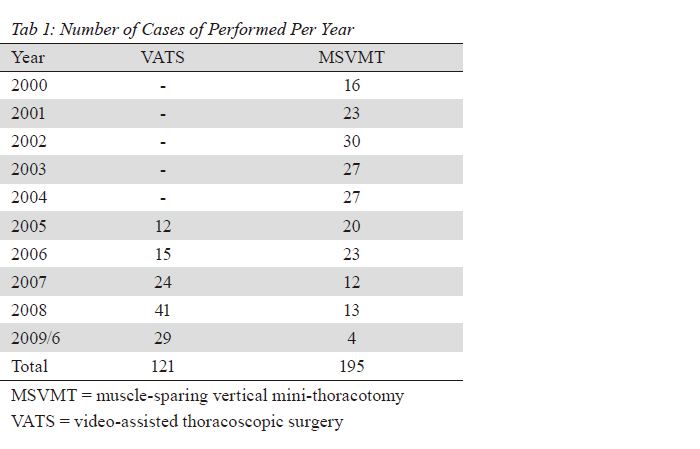
cancer survival. In our data, a total of 32 patients
underwent segmentectomy. There were 16 patients in
the VATS group and the other 16 in the MSVMT group.
In this study, we focus the analysis of the postoperative
complication difference between VATS and MSVMT,
not segmentectomy and lobectomy. We merged the data
of VATS lobectomy with VATS segmentectomy before
comparing the VATS group with MSVMT group on account of both lobectomy and segmentectomy being
considered as radical curative anatomic resection for
early lung cancer. We compared the data difference on
postoperative complications between the two groups and
found no significant difference (18.2% vs. 23.6%, p=0.255).
There was no surgical mortality in the VATS group and
only one conversion. We concluded that VATS lobectomy/
segmentectomy was a safe and technical feasible surgical
approach in our institute based on the present data.
Although the surgical risks of VATS lobectomy/
segmentectomy are considered to be acceptable, this new
operative approach has been adopted slowly over the past
decade. There seems to lack a generally accepted standard
procedure for VATS lobectomy/segmentectomy; however,
surgical techniques, differently modified, are proposed
from all over the world. The obstacles associated with
VATS included enigmatic technique skill, steep learning
curve, operative safety and oncologic concerns. There are
relatively few VATS reports for lung cancer in Taiwan to
date. We started VATS lobectomy/segmentectomy with
radical lymph node dissection for lung cancer in 2005. The
initial criteria for thoracoscopic surgery was limited to
small clinical early lung caner. Gradually, we extended the
indications of thoracoscopic surgery because of cumulative
experiences through time. In our institution to date,
patients considered appropriate for thoracoscopic approach include those tumors smaller than 5 cm in diameter
without central airway involvement or chest wall invasion.
Radical lymph node dissection should be routinely done for
definitively pathologic staging of mediastinal lymph node
status. Even those patients with single station N2 status
by PET-CT scan staging are considered candidates for
thoracoscopic surgery.
Many controversies regarding VATS approach face
further debates for consensus, which include the length
of utility thoracotomy, the application of rib spreader,
the usage of endoscopic instruments versus conventional
instruments and visualization through the incision or only
by the monitor. Even now, the thoracoscopic techniques
vary among nations, which may attribute, to some degree,
different results in the outcomes. We performed VATS
approach, which composed of video-monitor dependent
visualization, non-ribs spreading, and shorter-than-six cm
working wound length. A total of two to three chest wall
incisions were used in our institution.
Better quality of lymphadenectomy may lead to more
accurate tumor staging and therefore influence statistical
result. Patients with 15 or fewer mediastinal lymph nodes
dissected had worse survival outcome than those with more
than 15 (
20). Generally, we performed a radical mediastinal
lymph node dissection for all patients as much as we can.
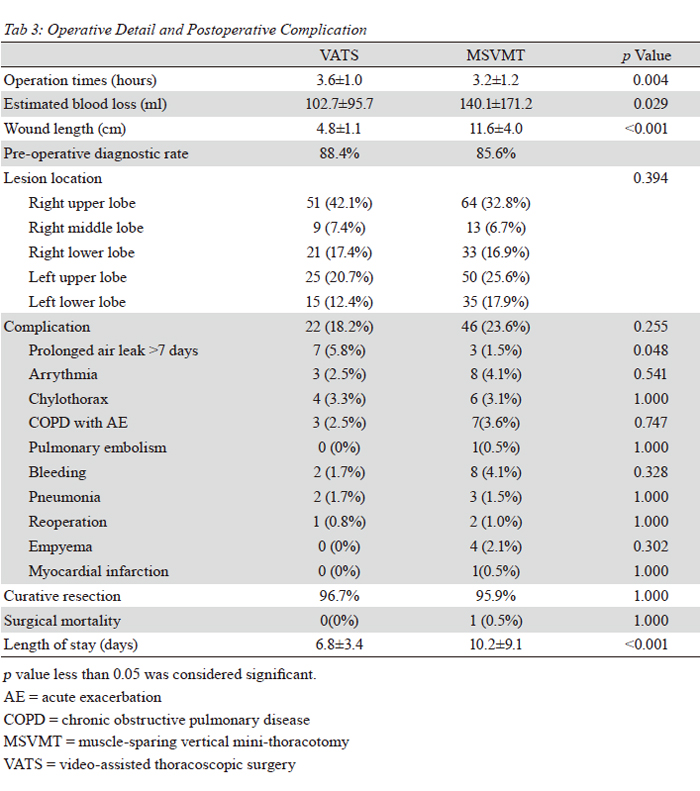
Our data demonstrates the number of dissected nodes
is smaller in the VATS group (p=0.005), but the mean
numbers of lymph node was larger than 15, which could
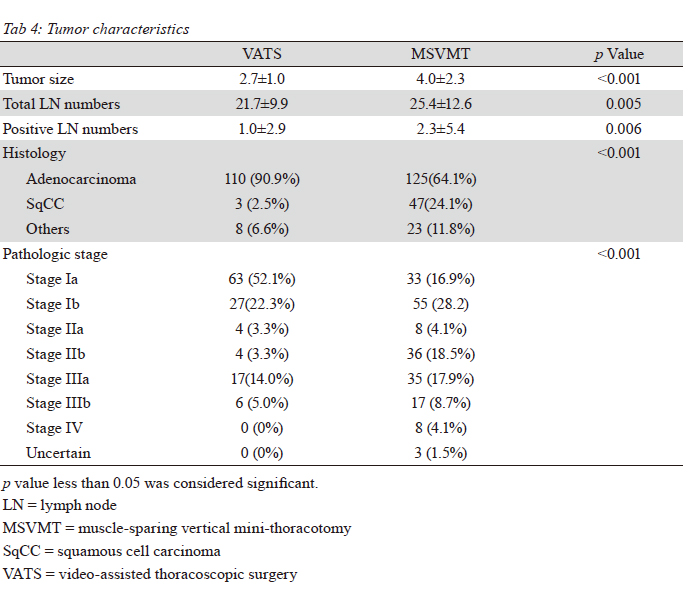
indicate the accurate tumor staging. We presumed that
patients in the VATS group had earlier stage lung cancer,
contributing to reduced numbers of lymph node. Of course,
it is impossible to discuss the technical impact of lymph
node dissection simply based on the numbers. As a matter
of fact, the technical quality of node dissection need to
be further analyzed according to long-term loco-regional
disease-free survival rate.
Adequate postoperative pain control has been known to
decrease postoperative pulmonary complications. Diminish
pain from chest wall incisions will improve the ability to
breathe deep, effectively cough and prevent correlative
atelectasis. VATS requires only two small skin incisions for
thoracoscopic insertion and utility thoracotomy window
without rib spreading, which lessen a lot of postoperative
pain (
21). The optimal postoperative pain control methods
for thoracoscopic surgery have been controversial. Epidural
anesthesia may be the most popular and well known means
of choice, however several associated complications have
been reported in literatures, such as nausea, vomiting,
hypotension, pruritus, constipation and technical related
complications (
22). Epidural analgesia is no longer used
in VATS group in our institution the potential risk could
be avoided. We prescribed oral non-steroid inflammatory drugs and oral opioids for postoperative pain control. Some
patients needed several additional shots of intravenous
opioids on postoperative first day. We didn’t compare
the pain scale between the two groups. In fact, epidural
anesthesia was only used in MSVMT group
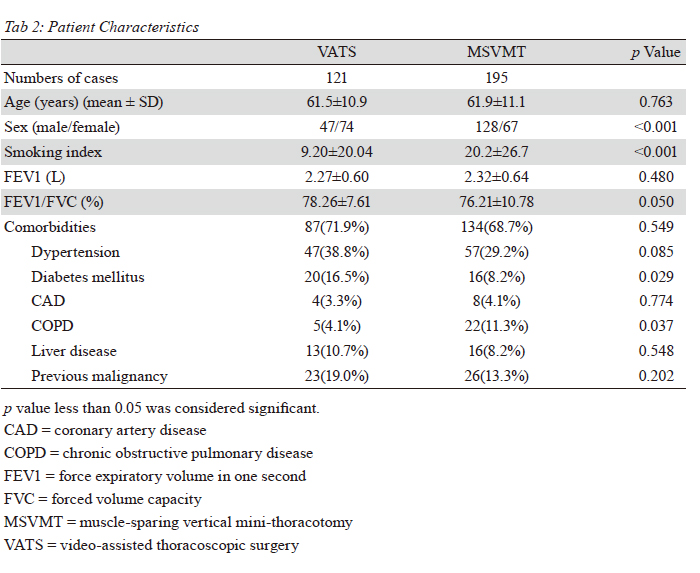
Thoracoscopic group had a significantly
predominant percentage in women, diabetes mellitus,
earlier stage, adenocarcinoma, less smoking index and
chronic obstructive pulmonary disease incidence in this
retrospective study. Limited by the retrospective nature,
the patient selection bias contributed to results. Definitive
conclusions regarding the VATS cannot be made on
account of the nature of this nonrandomized trial. We
showed our 10-year surgical experience of lung cancer
and the recognized advantages of VATS approach based
on our study are shorter hospital stay, less blood loss, less
epidural anesthesia necessaries, acceptable postoperative
complication and no surgical mortality. In conclusion, our
retrospective analysis demonstrated that VATS lobectomy/
segmentectomy is technically feasible, safe and holds more
comparative advantages to MSVMT approach.