Shedding light on the mechanisms of stent thrombosis with optical coherence tomography
Since the introduction of mechanical devices for the treatment of stenotic coronary artery disease, stent thrombosis (ST) has been an issue of major clinical concern. It is an uncommon but serious complication with high mortality and morbidity that almost always presents as death or myocardial infarction (MI), usually with electrocardiographic ST-segment elevation (1). Inadequate stent expansion and undersizing of a stent has been recognized as the main reason for acute and subacute ST (2) following stent implantation. Souteyrand et al. (3) have done a great job at confirming these findings with the use of optical coherence tomography (OCT). It is of interest that 69% of the OCT images were acquired during a deferred procedure, rather than in the acute presentation. Although it seems rational to focus on stabilizing a critically ill patient in the initial procedure, it would have been of interest to understand the reason for the delay in image acquisition, which has been shown to be safe (4). A median delay of 4.0 days is short, but it might have been of influence in retrieving the cause of the thrombosis. For instance, a small plaque rupture in neoatherosclerotic neointmia might be obscured after a few days by healing reaction and filling of the defect with fibrin deposition (5). Nevertheless, the authors managed to identify a morphological abnormality in 97% of the patients, which is remarkably high. In acute and subacute ST, stent malapposition and severe under expansion were the leading causes (48% and 26%, respectively). While in late and very late ST the rupture of a neoatherosclerotic plaque was identified as important factor (28%), next to malapposition (32%).
The concept of in-stent neoatherosclerosis is relatively new. One of the earliest studies (6) investigating late in-stent restenosis suggested delayed vascular healing due to chronic inflammation caused by the non-reabsorbable polymer of a metal stent leading to persistent fibrin deposition and restenosis as the main reason for late stent failure. Restenosis and late ST were considered two separate entities. Later studies (7-10), however, have shown that neoatherosclerosis is a major contributor to late stent failure. The paradigm is now shifting to a concept where restenosis and ST are inextricably linked (Figure 1). It has become obvious that thin cap fibroatheromas (TCFA) within a neoatherosclerotic plaque can rupture, causing thrombus and acute occlusion of a coronary artery. This is clinically relevant, because it means that stented coronary segments are still at risk for atherosclerosis and intensive secondary preventive therapy is of major importance.
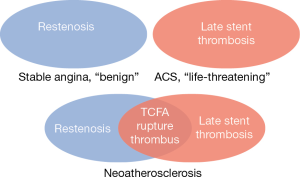
While rupture of a neoatherosclerotic plaque was seen as second most reason for late and very late ST, strut malapposition was the most frequently observed abnormality throughout all ST types. Malapposition, as well as undersizing, are often the result of poor stent selection or inadequate lesion preparation or post-dilation, possibly caused by angiographic misinterpretation. Angiography is unquestionably the golden standard for guidance of percutaneous coronary interventions, but it suffers from artefacts that are directly related to its 2D-representation of the coronary lumen, like foreshortening or vessel overlap. This could not only affect stent implantation, but also has its impact on retrieving the possible cause of ST, which is often missed on angiographic images. This is well illustrated in the current study. With its detailed images of both the coronary lumen and stent struts, OCT was able to reduce the number of ‘unidentified’ mechanisms of ST from 48% to 13%.
The solution to resolve these shortcomings seems straightforward. Several studies have demonstrated improved outcome after invasive imaging guided stent implantation, mainly intravascular ultrasound (IVUS) at this point in time. With knowledge about a vessel’s true dimensions that are not being influenced by the previously mentioned angiographic artefacts, undersizing should be easier to avoid. Furthermore, lesion preparation and assessment for the need of post-dilation can be performed with great detail and should result in less strut malapposition and thus less ST. Large randomized clinical trials that underline these hypotheses, however, are still lacking. Hopefully the DOCTOR-trial (11) will shed some light on this much debated topic.
An often heard criticism about invasive imaging was that the geographical position of cross-sectional OCT or IVUS images gathered was often difficult to quickly and reliable match on angiographic views, especially in absence of so-called anatomical landmarks (e.g., side branches or stent edges). Systems that can co-register both angiographic and cross-sectional images have already been introduced (12,13) and will help to negate this remaining challenge.
Another remaining challenge is the definition of clear indications for the use of invasive coronary imaging. At this point, there are no studies yet that provide clear recommendations. The ESC guidelines on myocardial revascularization (14), however, already state that IVUS and/or OCT should be considered to detect stent-related mechanical problems. The current study by Souteyrand et al. demonstrated that such mechanical issues were presented in 97% of the cases with ST. Therefore, we believe that intracoronary imaging is a must for every case of ST in the modern catheterization laboratory.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Feng Zhang (Department of Cardiology, Zhongshan Hospital of Fudan University, Shanghai, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med 2007;356:998-1008. [Crossref] [PubMed]
- Hall P, Colombo A, Almagor Y, et al. Preliminary experience with intravascular ultrasound guided Palmaz-Schatz coronary stenting: the acute and short-term results on a consecutive series of patients. J Interv Cardiol 1994;7:141-59. [Crossref] [PubMed]
- Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J 2016;37:1208-16. [Crossref] [PubMed]
- van der Sijde JN, Karanasos A, van Ditzhuijzen NS, et al. Safety of optical coherence tomography in daily practice: a comparison with intravascular ultrasound. Eur Heart J Cardiovasc Imaging 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Gonzalo N, Tearney GJ, van Soest G, et al. Witnessed coronary plaque rupture during cardiac catheterization. JACC Cardiovasc Imaging 2011;4:437-8. [Crossref] [PubMed]
- Virmani R, Liistro F, Stankovic G, et al. Mechanism of late in-stent restenosis after implantation of a paclitaxel derivate-eluting polymer stent system in humans. Circulation 2002;106:2649-51. [Crossref] [PubMed]
- Tanimoto S, Aoki J, Serruys PW, et al. Paclitaxel-eluting stent restenosis shows three-layer appearance by optical coherence tomography. EuroIntervention 2006;1:484. [PubMed]
- Higo T, Ueda Y, Oyabu J, et al. Atherosclerotic and thrombogenic neointima formed over sirolimus drug-eluting stent: an angioscopic study. JACC Cardiovasc Imaging 2009;2:616-24. [Crossref] [PubMed]
- Kang SJ, Mintz GS, Park DW, et al. Tissue characterization of in-stent neointima using intravascular ultrasound radiofrequency data analysis. Am J Cardiol 2010;106:1561-5. [Crossref] [PubMed]
- Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 2015;36:2147-59. [Crossref] [PubMed]
- Meneveau N, Ecarnot F, Souteyrand G, et al. Does optical coherence tomography optimize results of stenting? Rationale and study design. Am Heart J 2014;168:175-81.e1-2.
- Tu S, Holm NR, Koning G, et al. Fusion of 3D QCA and IVUS/OCT. Int J Cardiovasc Imaging 2011;27:197-207. [Crossref] [PubMed]
- Carlier S, Didday R, Slots T, et al. A new method for real-time co-registration of 3D coronary angiography and intravascular ultrasound or optical coherence tomography. Cardiovasc Revasc Med 2014;15:226-32. [Crossref] [PubMed]
- Authors/Task Force members, Windecker S, Kolh P, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541-619. [Crossref] [PubMed]


