A randomized, open, multicenter clinical study on the short course of intravenous infusion of 750 mg of levofloxacin and the sequential standard course of intravenous infusion/oral administration of 500 mg of levofloxacin for treatment of community-acquired pneumonia
Introduction
Community-acquired pneumonia (CAP) is a common infection in the community with very high incidence and mortality (1). The incidence of CAP is approximately 1.5–14.0 cases/1,000/year, which, however, varies with different regions, seasons and populations. The mortality is up to 4.0–18.0% for inpatients and 50% for patients in intensive care unit (ICU) (1). These give rise to the huge medical costs. In the United States, direct medical cost for CAP each year is about 8.4 to 9.7 billion dollars (2,3), which is overwhelmingly unbearable for developing countries lack of medical resources. Streptococcus pneumoniae (S. pneumoniae) and Mycoplasma pneumoniae (M. pneumoniae) are the most common pathogens of CAP (1). The growing drug resistance of these two pathogens has become an issue of concern to clinicians. The epidemiological investigation on CAP nationwide in China from 2003 to 2004 (4) shows 22.2% of S. pneumoniae is insensitive to penicillin (MIC ≥0.12 µg/mL), 79.4% to azithromycin and only 6.3% to levofloxacin. The incidence of M. pneumoniae resistant to macrolides is more serious and growing year by year. Zhao et al. (5) reported the resistant rate of M. pneumoniae to macrolides increased from 68.9% in 2008 to 97.0% in 2012 in China. However, M. pneumoniae is sensitive to fluoroquinolones. In front of growing resistance, how to maximize the ratio of efficacy to resources is the eternal subject of clinicians and also our social responsibility to improve human health.
The exploration of the potential of existing drugs is undoubtedly more efficient and affordable compared to the development of new medicines. How to optimize the existing regimens for better efficacy or shorter course and how to minimize the emergence of drug resistance and reduce adverse reactions are issues worthy of further discussion. Levofloxacin is a commonly used fluoroquinolones agent for the treatment of CAP in many countries. Generally, the course of treatment ranges from 7 to 14 days (6,7). Levofloxacin is a concentration-dependent antibacterial agent with its therapeutic outcome closely related to the ratio of the area under the concentration-time curve to the minimum inhibitory concentration (AUC/MIC) for the organism. Also, a high ratio of peak plasma concentration (Cmax) to MIC contributes to the prevention of drug resistance (8). Increasing the dose of these kinds of antimicrobial agents, such as levofloxacin 750 mg once daily, in a safe range may shorten the duration of treatment, and inhibit drug resistance, as well as save medical costs without compromising clinical outcomes. Pharmacokinetic/pharmacodynamic (PK/PD) studies show that compared with levofloxacin 500 mg, levofloxacin 750 mg can increase plasma AUC and Cmax values by 0.5 fold (9,10). American scholars reported (11) the 5-day course of levofloxacin 750 mg per day was as effective as the 10-day course of levofloxacin 500 mg per day for the treatment of CAP with no significant increase in side effects. Currently, few data on the short-course, high-dose levofloxacin regimen for the treatment of CAP in Chinese population are available. Therefore, we intended to expand the sample size and conducted this prospective, open, multicenter clinical trial to investigate whether the short course regimen (levofloxacin 750 mg/day, intravenous infusion for 5 days) was as effective and safe as the conventional regimen (levofloxacin 500 mg/day, intravenous/oral sequential therapy for 7–14 days) for the treatment of CAP in Chinese population.
Methods
Study design
Sample size estimate
According to reference (11), assume the clinical efficacy rate of levofloxacin 750 mg group as 88% and the clinical efficacy rate of levofloxacin 500 mg group as 86%, 330 patients are eligible to be assessed, in which one-tailed significance level was 2.5%, and power of test was 90%, and margin was 10%. If drop-out rate was 20%, 414 patients would be enrolled in this study.
Detailed study design
This was a randomized, open labeled, active treatment-controlled, multi-center and non-inferiority clinical study conducted in China. After the approval of the ethics committee of People’s Liberation Army General Hospital (EC approval No: 2012018), CAP patients were enrolled from 17 large hospitals in Beijing, Shanghai, Zhejiang, Hunan and Guangzhou in Chinese mainland from November 2012 to July 2014. Study plan was not changed after initiation of the study. All patients were required to sign the informed consent after the enrollment and before the administration. The block randomization was carried out by study center (block size =4). The eligible subjects were randomized into levofloxacin 750 mg group or levofloxacin 500 mg treatment group at the ratio of 1:1. Both study drug and control drug were provided by Daiichi Sankyo Company Limited. Study group (750 mg group) was intravenous infusion of levofloxacin, 750 mg/d, for 5 days, while control group (500 mg group) was intravenous/oral administration of levofloxacin, 500 mg/d, for 7–14 days. When the symptoms were significantly improved with body temperature decreased and white blood cell count reaching to the normal level and the patient could orally take drugs, intravenous infusion would be changed to sequential oral administration. If the patient needed to withdraw this study because of the poor therapeutic effect, drug-related side effects, or certain special pathogens isolated, the best alternative treatment was selected according to the specific conditions of the patient by the investigator.
Inclusion and exclusion criteria
Adult CAP patients (≥18 years) or those within 48 hours of admission including newly diagnosed patients, subsequent visit and referral patients due to the treatment failure with non-fluoroquinolone agents were enrolled. The diagnostic criteria of CAP were referred to the criteria in the Practice of Internal Medicine (Edition 11) (12). Inclusion criteria included: (I) chest X-ray examination revealed new inflammatory infiltration; (II) presence of ≥2 of the following manifestations: fever (axillary temperature ≥37 °C or oral temperature ≥37.5 °C or rectal temperature ≥38 °C), peripheral blood white blood cell count >10×109/L or neutrophil percentage >70%, cough, chest pain, wet rale in the lung by auscultation; (III) CURB-65 score: 0–2 points. Patients with any of the following conditions were excluded: (I) pregnant women, lactating women or female patients trying to conceive during the study; (II) a history of epilepsy; (III) a history of quinolone-induced tendon lesions; (IV) previous allergic or intolerant history to levofloxacin or other quinolones; (V) those who had used non-quinolones within 72 hours prior to the administration of the study drug and the condition had been improved; (VI) those with serious cardiac, hepatic (ALT or AST >2× upper limit of normal), renal (serum creatinine >1.5× upper limit of normal) diseases or other diseases as the investigator considered the patients were not eligible to participate in this trial; (VII) prolonged QT interval in ECG at enrollment or a past history of the same disease; (VIII) patients with declined WBC count in peripheral blood (WBC <4.0×109/L) or neutropenia (neutrophils <2.0×109/L); (IX) patients who had used any of the study drugs within 4 weeks before administration in this study; (X) at the investigator’s discretion, the subject had any kind of the clinical diseases or abnormal conditions that might threat the subject’s safety or compromise the quality of study data.
The relevant clinical data (age, gender, underlying diseases, symptoms or signs, clinical outcomes, etc.), laboratory tests (blood routine, C-reactive protein, liver and kidney function, electrolytes, etc.), electrocardiogram, chest imaging and other information of all the enrolled patients were collected for data entry.
Microbiologic detection methods and diagnostic criteria
The sputum specimens were collected within 24 hours after the enrollment and before administration (visit 1). The specimens were smeared for Gram stain, and screened with microscopic examination for eligible specimen (<10 squamous epithelial cells/low-power field, >25 polymorphonuclear leukocytes/low-power field, or squamous epithelial cells: polymorphonuclear leukocytes <1:2.5). Then, bacterial culture, isolation, identification and antimicrobial sensitivity test were performed with the eligible specimens. If the bacterial culture result was positive, the culture would be repeated on the first day after the end of the treatment (EOT, visit 4) and 9–15 days after the end of the treatment (visit 5). Blood samples were taken for culture for patients with fever (body temperature >38.5 °C). If the culture result was positive, blood culture would be repeated on the first day after the end of the treatment (visit 4) and 9–15 days after the end of the treatment (visit 5). Eight to ten milliliter of midstream urine specimen was collected from each enrolled patients before the administration of antimocrobial therapy to detect the urinary antigen of S. pneumoniae (using BINAX NOW Streptococcus pneumoniae urinary antigen test kit provided by BINAX Company with immune chromatography).
Positive pathogen diagnostic criteria: (I) one or more strains of bacteria cultured in an eligible respiratory specimen; (II) significant pathogen(s) in blood culture; (III) S. pneumoniae urinary antigen test positive.
Clinical and microbiological efficacies evaluation
Criteria for clinical efficacy evaluation
At the end of treatment (EOT) (visit 3 of 750 mg and 4 of 500 mg arms respectively), the clinical efficacy was evaluated as the primary endpoints. The detailed criteria were as follows: Cured: resolution of pretreatment abnormal clinical signs and symptoms, with no further antimicrobial therapy required for CAP. Improved: clinical findings subsided significantly but with incomplete resolution of clinical evidence of infection in patients who required no further antimicrobial therapy for CAP. Failed: no significant response to therapy or an incomplete response that requires additional antimicrobial therapy for CAP. NA: clinical judgment of cure, improvement, or failure could not be made because of certain reasons (e.g., lost to follow-up, lack of information, medication for less than 3 days, etc.). Clinical efficacy was defined as clinical cured + clinical improved.
Criteria for microbiological efficacy evaluation
At the EOT (visit 3 of 750 mg and 4 of 500 mg arms respectively), the microbiological response was evaluated. The detailed criteria were as follows: Eradication/presumed eradication: all pathogens isolated before the treatment did not exist; or, clinical signs/symptoms had improved significantly, with no sputum specimen could be collected, and investigators speculated pathogens isolated before the treatment had been eradicated. Partial eradication: a variety of pathogens were isolated before the treatment and one pathogen isolated was eradicated after the treatment while another (or several) pathogen persisted. Uneradicated/presumed existence: persistence of the pathogens isolated before the treatment; or, no response to clinical therapy, and it could be speculated that the pathogens isolated before the treatment persisted. New infections: the emergence of pathogen(s) different from the original pathogen(s) isolated before the treatment, with the signs and symptoms of pneumonia. Recurrence: the emergence of the pathogen(s) isolated before the treatment within 14 days after the end of the treatment, with the signs and symptoms of CAP. Unable to evaluate: the evaluation of microbiological response could not be made because of certain reasons (e.g., lost to follow-up, lack of information, medication for less than 3 days, etc.). Microbiological efficacy was defined as eradication, presumed eradication and new infections.
Safety evaluation
In this study, any adverse medical events occurred in subjects after study drug administration are considered adverse events. Adverse events included clinical adverse events and laboratory abnormalities. Among laboratory tests, ALT or AST >2× upper limit of normal and serum creatinine >1.5× upper limit of normal were defined abnormal. Other tests below or above the normal range in the laboratories of each center (including abnormalities with no clinical significance and abnormalities with clinical significance) were recorded as laboratory abnormalities. Abnormalities (including abnormalities with no clinical significance and abnormalities with clinical significance) in ECG reports were recorded as ECG abnormalities. The severity of adverse events was classified by the severity and the severity was evaluated as mild (mild, transient and tolerable signs or symptoms), moderate (signs or symptoms that impact daily activities) and severe (signs or symptoms that disable the subject from working or performing daily activities, and may be systemic or in need of medical evaluation and/or treatment). Investigators make judgements of study drug relevance to all adverse events. The relationship between adverse events and drug is categorized as: definitely related: the use of the study drug has a reasonable time relationship and clinical events cannot be explained by underlying diseases or other drugs (including laboratory abnormalities); adverse events disappear after drug withdrawal. The adverse events must be pharmacologically or phenomenologically known reaction. Provocation test result is positive. Probably related: the use of the study drug has a reasonable time relationship. Clinical events cannot be possibly explained by underlying diseases or other drugs. There are reasonable clinical events after drug withdrawal (including laboratory abnormalities). No provocation test is needed for confirmation. Possibly related: the use of the study drug has a reasonable time relationship. Clinical events may be possibly explained by underlying diseases or other drugs (including laboratory abnormalities). Drug withdrawal information may be “no” or “not clear”. Possibly unrelated: the use of the study drug does not have a reasonable time relationship. Clinical events can be possibly explained by underlying diseases or other drugs (including laboratory abnormalities). Unrelated: the use of the drug is definitely unrelated to clinical events (including laboratory abnormalities).
Statistics
Analysis set
Subjects who received at least 1 dose of study medication and had a visit post the treatment were included in the intent-to-treat (ITT) population. Subjects who completed the entire course of the study and had no significant violation to the protocol were included in the per-protocol set (PPS) population in this study. Efficacy analysis was carried out in the ITT population and the PPS population. All patients who received at least 1 dose of study medication were included in the safety set (SS) population. Safety analysis was carried out in safety population. ITT population was used for the analysis of the primary endpoint.
Statistical methods
SAS 9.4 software was used for the statistic description and analysis of the data. The description of quantitative indicators included the total number of cases, mean, standard deviation, median, interquartile range, etc. The description of categorical indicators included the number of cases and the percentage. The clinical efficacy rate was compared between the two groups of patients at EOT using the chi-square test. The difference in clinical efficacy rate and the 95% CI at EOT were calculated between the two groups of patients. When the lower limit of the 95% CI for clinical efficacy rate difference at EOT in ITT analysis was above −10%, it could be determined that the clinical efficacy rate in the study group was non-inferior to that in the control group. Clinical relapse rates were compared using Fisher’s exact test. Among the demographic data, the quantitative variables were compared using paired t-test and the categorical variables using chi-square test/Fisher’s exact test. The changes of body temperature and the incidence of adverse events were compared using chi-square test. The judgment criterion of P<0.05 was statistically significant.
Results
General clinical information
A total of 457 patients were enrolled. Two hundred and twenty-eight subjects were randomized into levofloxacin 750 mg group and 229 into levofloxacin 500 mg group. After removal of seven patients who were unable to be evaluated due to incomplete data and two patients who did not meet the inclusion criteria, a total of 448 patients entered the ITT groups, including 221 in 750 mg group and 227 in 500 mg group. After removal of twenty one patients who did not meet the eligibility criteria or exclusion criteria; violated the plan; treatment duration was less than three days; drug administration was inconsistent with group allocation, a total of 427 patients entered the PPS groups, including 208 in 750 mg group and 219 in 500 mg group. A total of 457 patients entered the SS groups, including 228 in 750 mg group and 229 in 500 mg group (Figure 1). The gender, weight, underlying disease and other characteristics, except age, in ITT population or PPS population were not significantly different between the two groups, P>0.05, except the age of two groups, P=0.041 and P=0.081 in ITT and PPS populations (Table 1). About 40% patients had non-quinolone antibacterial drug treatment prior to randomization to the groups (Table 2). The mean hospital stay of levofloxacin 750 mg group and 500 mg group was 8.42±4.01 and 10.19±4.47 days, respectively (P=0.0013). Within 15-day follow-up period after EOT, no death occurred in both groups.
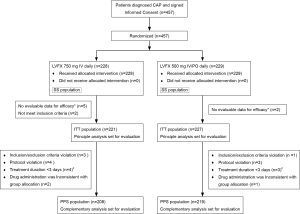
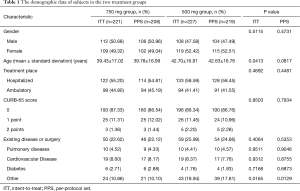
Full table
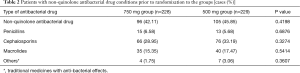
Full table
Clinical efficacy
At the EOT, clinical efficacy rate difference and its 95% CI between levofloxacin 750 mg group and levofloxacin 500 mg group was −2.87 (−7.64, 1.90), and lower limit of confidence interval was greater than −10 and non-inferiority test for ITT, P value >0.001. For treating CAP, clinical efficacy rate of 5-day short-course therapy in levofloxacin 750 mg group showed non-inferiority compared with that of 7–14-day sequential therapy in levofloxacin 500 mg group (Table 3). Subjects in ITT population were followed up 9–15 days after the EOT (visit 5). The rate of clinical relapse was 0.49% (1/205) in 750 mg group and 1.41% (3/213) in 500 mg group. The difference was not statistically significant between the two groups (P=0.6235).

Full table
Microbiological efficacy
The bacterial isolation rates were 8.14% (18/221) and 7.49% (17/227) in ITT population of 750 and 500 mg groups, respectively. In ITT population, the detection rate of the urinary antigen of S. pneumoniae was 7.69% (17/221) in 750 mg group and 7.21% (16/227) in 500 mg group. At the EOT, all isolates were eradicated/presumably eradicated in both groups. The microbiological efficacy was 100% in the two groups.
Clinical resolution
The median time for fever resolved was 4 days in both 750 mg group and in 500 mg group among ITT population. The differences in changes of body temperature and WBC count were not significant (Table 4).

Full table
Safety
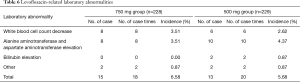
Two hundred and two-eight patients in levofloxacin 750 mg group and 229 patients in levofloxacin 500 mg group were evaluated for safety. The incidences of adverse events were 24.56% (56 cases, 93 cases times) and 18.78% (43 cases, 70 cases times) respectively. And the incidences of drug-related adverse events were 18.42% (42 cases, 60 case times) and 13.54% (31 cases, 48 cases times) respectively. The most common drug-related adverse events in 750 mg group and 500 mg group were shown in Tables 5 and 6. The difference in adverse events was not statistically significant between the two groups (P>0.05). In this study, three subjects in 750 mg group developed drug-related cardiac side effects, which were palpitations, I degree atrioventricular block and premature ventricular contractions. One subject in 500 mg group had drug-related electrocardiogram abnormalities (short PR interval, flat T wave, counterclockwise rotation). Palpitations were improved after drug withdrawal. Other adverse events were mild and did not need any treatment. Severe adverse events were reported in three cases. Among them, 1 subject (0.44%) in levofloxacin 750 mg group had high fever at study entry, and the body temperature did not drop after 3 days of treatment. Then the patient was admitted to another hospital for treatment. Two patients (0.87%) in 500 mg group developed severe adverse events, one with bone metastasis of prostate cancer detected after study entry, and the other with uterine myoma detected after study entry who was hospitalized for treatment during the follow-up period. Three cases of severe adverse events were considered unrelated to the study drug.
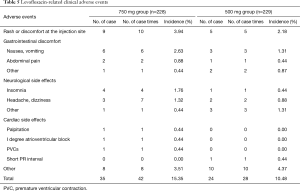
Full table
Full table
Discussion
Levofloxacin is a concentration-dependent antibacterial agent. Pharmacokinetic/pharmacodynamic (PK/PD) studies have shown that increased dose gives rise to higher plasma concentration and alveolar epithelial lining fluid concentration as well as enhanced antibacterial activity (13,14), which is beneficial for the treatment of respiratory tract infections. The study of Gotfried (13) showed when levofloxacin was administered once daily for 5 days, the plasma concentrations of levofloxacin were respectively 12.0±3.0 µg/mL in 750 mg group and 5.3±1.2 µg/mL in 500 mg group 4 hours after administration. And the alveolar epithelial lining fluid concentrations were 22.1±14.9 µg/mL in 750 mg group and 9.9±2.7 µg/mL in 500 mg group respectively. Twenty-four hours after the administration, the plasma concentrations were 1.7±1.1 µg/mL in 750 mg group and 0.6±0.1 µg/mL in 500 mg group respectively. And the alveolar epithelial lining fluid concentrations were 1.5±0.8 µg/mL in 750 mg group and 0.7±0.4 µg/mL in 500 mg group separately. Lister (14) reported levofloxacin at the dose of 750 mg could eradicate S. pneumoniae which MIC were 2.6 and 3.2 µg/mL, slightly higher than 2 µg/mL, while levofloxacin at the dose of 500 mg could not eliminate the number of bacteria to <104 CFU/mL. S. pneumoniae is one of the most common pathogens of CAP. It is recommended to increase PK/PD parameters, Cmax/MIC ≥5, AUC/MIC >30 to achieve favorable antibacterial effect (15,16). American scholar Frei (17) studied the probability of target attainment (PTA) of levofloxacin against S. pneumoniae with the target of AUC/MIC >30 with Monte Carlo simulation. The results showed PTA was 99% in levofloxacin 750 mg group and 90% in 500 mg group, suggesting the PK/PD parameters of levofloxacin at the dose of 750 mg are superior to those at the dose of 500 mg, and more suitable for the treatment of pulmonary infections. The epidemiological investigation of CAP in China (4) showed: the MIC50 of levofloxacin for S. pneumoniae was 1 µg/mL and MIC90 was 2 µg/mL. Levofloxacin at the dose of 750 mg would be more favorable for the treatment of pulmonary infection due to S. pneumoniae.
Up till now, there are four published multicenter, randomized clinical studies of the treatment for CAP by using levofloxacin to compare clinical and microbiological efficacy and adverse events of high dose (750 mg/day) and short course treatment and the conventional dose (500 mg/day) therapy (11,18-20). The result shows with equivalence of the clinical and microbiological efficacy, the occurrence of adverse events is equivalent. The study published by Shorr AF in 2005 was only aimed at the people over 65 years old (19); another study published by Shorr AF in 2006 showed that the improvement ratio of fever and purulent sputum from the group of 750 mg was higher than that from the group of 500 mg after the treatment of three days (18); There is only one study whose study population was Chinese (20). But the sample size was relatively small and only 241 patients were involved. We increased the sample size of Chinese population with CAP and 457 patients were involved. The result showed that the clinically effective rates were 91.40% and 94.27% in 750 mg high-dose regimen and 500 mg conventional regimen respectively. And the bacterial eradication rate was 100.0% in the two groups. The incidences of drug-related adverse events were 18.42% and 13.54% respectively. At the investigator’s discretion, three cases of serious adverse events were unrelated to study drug. The non-inferiority hypothesis was tenable (non-inferiority margin 10%), which was similar to the findings by other reports (11,18). Although around 40% patients in our study had other non-quinolone antibacterial agents treatment prior to enrollment, the selected patients were those experienced non-quinolones antibiotic treatment failure. We consider it will not affect the final efficacy judgement of levofloxacin. Additionally, levofloxacin 750 mg daily dosage was 1.5-fold of levofloxacin 500 mg daily dosage, but the therapeutic durations of 750 mg regimen was half of 500 mg regimen, 4.86 vs. 10.35 days. Moreover, the mean drug exposure was 3,641.4 mg in 750 mg group and 5,169.6 mg in 500 mg group, 30% of total drug exposure reduction in 750 mg group. The results of similar efficacy and halved therapeutic duration encourage us to further study the pharmaceutical economics for CAP treatment in order to quantify economic social benefit caused by shortening the treatment course and saving medical resources. In addition, short-course levofloxacin regimen can significantly reduce total drug exposure, which helps reduce the growth of bacterial resistance, though common respiratory pathogens are still sensitive to levofloxacin currently (4,5). In short, the optimization of currently marketed drug regimen may well be a drug development direction to efficiently and effectively using the limited resources.
This study has some limitations: (I) the enrolled patients were diagnosed with mild to moderate CAP with no critically ill condition, and thus the results cannot be generalized to cover all patients with CAP. Although the studies of American scholar Dunbar (11) showed that for CAP patients in PSI stratum IV, the clinically effective rate in levofloxacin 750 mg (for 5 days) was comparable (92.6% vs. 84.4%, 95% confidence interval −26.1% to 9.6%) to that in levofloxacin 500 mg group (for 10 days). However, the sample size was too small with 27 and 32 cases in the two groups respectively, so the conclusion cannot be generalized to all patients with severe CAP and further clinical studies are needed; (II) in this study, bacterial culture positive rate was low, 8.14% in 750 mg group and 7.49% in 500 mg group respectively. The reference value of microbiological evaluation is limited. Therefore, we did not perform the statistical analysis of bacteriological efficacy because of limited pathogens isolated. The main reason for the low rate of bacterial isolation could be more than 40% of patients treated antibiotics (non-fluoroquinolone) before the enrollment; (III) the detection of atypical pathogens was not performed in this study. Atypical pathogens, especially M. pneumoniae, are common pathogens of CAP and the detection rate in CAP is about 20% in China. M. pneumoniae is sensitive to levofloxacin in China, suggesting the clinical efficacy is favorable. However, M. pneumoniae related efficacy could not be analyzed in this study; (IV) although this study passed Central Ethical Review of General Hospital of PLA, the registration has not been performed in the clinical study websites. In addition, an open-label design instead of a blind design adopted in this study may lead to certain biases in the result judgment. However, besides the subjective symptoms indicators in the evaluation, objective indicators from the lab examination such as white blood cells, C-reactive protein, and chest X-rays help to reduce the bias from patients or investigators; (V) this study is a clinical research. Relative stringent exclusion criteria were set in consideration of the patient’s safety and a part of CAP patients were excluded. As a result, the patients included in the study are relatively young (the mean age is approximately 40) and have few underlying diseases (22.62–25.99%). Although more than half of the patients were hospitalized (55.20–58.59%), no death occurred in the clinical study period. Therefore, clinical adverse events might be less frequent on some level. Further studies need to be performed on evaluation of clinical efficacy and safety for the patients not enrolled in the groups; (VI) for certain self-limiting pathogens, such as CAP caused by virus, the difference of the evaluation time points may affect the clinical efficacy. As the evaluation was made at EOT for this study, the treatment duration (7–14 days) of levofloxacin 500 mg group was slightly longer than that of the 750 mg group. Efficacy evaluation may be more beneficial to the 500 mg group.
Conclusions
In summary, the 5-day regimen of levofloxacin 750 mg of IV levofloxacin per day is non-inferior to the conventionally sequential 7–14-day regimen of the levofloxacin 500 mg IV/PO per day for the treatment of mild to moderate CAP in Chinese population. The short course regimen allows the reduction of mean total dose of antimicrobial drug and well tolerated. It is worthy of promotion in clinical application.
Acknowledgements
The authors would like to thank all study participants and their families for their cooperation.
The study was funded by Daiichi Sankyo Co., Ltd.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the ethics committee of People’s Liberation Army General Hospital (EC approval No: 2012018). All patients were required to sign the informed consent after the enrollment and before the administration.
References
- Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet 2015;386:1097-108. [Crossref] [PubMed]
- Niederman MS, Ahmed QA. Community-acquired pneumonia in elderly patients. Clin Geriatr Med 2003;19:101-20. [Crossref] [PubMed]
- Arnold FW, Ramirez JA, McDonald LC, et al. Hospitalization for community-acquired pneumonia: the pneumonia severity index vs. clinical judgment. Chest 2003;124:121-4. [Crossref] [PubMed]
- Liu Y, Chen M, Zhao T, et al. Causative agent distribution and antibiotic therapy assessment among adult patients with community acquired pneumonia in Chinese urban population. BMC Infect Dis 2009;9:31. [Crossref] [PubMed]
- Zhao F, Liu G, Wu J, et al. Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob Agents Chemother 2013;57:1521-3. [Crossref] [PubMed]
- Frank E, Liu J, Kinasewitz G, et al. A multicenter, open-label, randomized comparison of levofloxacin and azithromycin plus ceftriaxone in hospitalized adults with moderate to severe community-acquired pneumonia. Clin Ther 2002;24:1292-308. [Crossref] [PubMed]
- Zhang YY, Huang HH, Ren ZY, et al. Clinical evaluation of oral levofloxacin 500 mg once-daily dosage for treatment of lower respiratory tract infections and urinary tract infections: a prospective multicenter study in China. J Infect Chemother 2009;15:301-11. [Crossref] [PubMed]
- Madaras-Kelly KJ, Demasters TA. In vitro characterization of fluoroquinolone concentration/MIC antimicrobial activity and resistance while simulating clinical pharmacokinetics of levofloxacin, ofloxacin, or ciprofloxacin against Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2000;37:253-60. [Crossref] [PubMed]
- Lister PD. Pharmacodynamics of 750 mg and 500 mg doses of levofloxacin against ciprofloxacin-resistant strains of Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2002;44:43-9. [Crossref] [PubMed]
- Noreddin AM, Hoban DJ, Zhanel GG. Comparison of gatifloxacin and levofloxacin administered at various dosing regimens to hospitalised patients with community-acquired pneumonia: pharmacodynamic target attainment study using North American surveillance data for Streptococcus pneumoniae. Int J Antimicrob Agents 2005;26:120-5. [Crossref] [PubMed]
- Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis 2003;37:752-60. [Crossref] [PubMed]
- Chen HZ. Practice of internal medicine (in Chinese) 11th ed. Beijing: People’s Medical Publishing House, 2001:1538-51; 1566-8.
- Gotfried MH, Danziger LH, Rodvold KA. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 2001;119:1114-22. [Crossref] [PubMed]
- Lister PD. Pharmacodynamics of 750 mg and 500 mg doses of levofloxacin against ciprofloxacin-resistant strains of Streptococcus pneumoniae. Diagn Microbiol Infect Dis 2002;44:43-9. [Crossref] [PubMed]
- Cao G, Zhang J, Wu X, et al. Pharmacokinetics and pharmacodynamics of levofloxacin injection in healthy Chinese volunteers and dosing regimen optimization. J Clin Pharm Ther 2013;38:394-400. [Crossref] [PubMed]
- Nightingale CH, Grant EM, Quintiliani R. Pharmacodynamics and pharmacokinetics of levofloxacin. Chemotherapy 2000;46 Suppl 1:6-14. [Crossref] [PubMed]
- Frei CR, Burgess DS. Pharmacodynamic analysis of ceftriaxone, gatifloxacin,and levofloxacin against Streptococcus pneumoniae with the use of Monte Carlo simulation. Pharmacotherapy 2005;25:1161-7. [Crossref] [PubMed]
- Shorr AF, Khashab MM, Xiang JX, et al. Levofloxacin 750-mg for 5 days for the treatment of hospitalized Fine Risk Class III/IV community-acquired pneumonia patients. Respir Med 2006;100:2129-36. [Crossref] [PubMed]
- Shorr AF, Zadeikis N, Xiang JX, et al. A multicenter, randomized, double-blind, retrospective comparison of 5- and 10-day regimens of levofloxacin in a subgroup of patients aged > or =65 years with community-acquired pneumonia. Clin Ther 2005;27:1251-9. [Crossref] [PubMed]
- Zhao X, Wu JF, Xiu QY, et al. A randomized controlled clinical trial of levofloxacin 750 mg versus 500 mg intravenous infusion in the treatment of community-acquired pneumonia. Diagn Microbiol Infect Dis 2014;80:141-7. [Crossref] [PubMed]


