Relapsed small-cell lung cancer: platinum re-challenge or not
Small-cell lung cancer (SCLC) is an aggressive pulmonary tumor characterized by a rapid doubling time, high growth fraction, and the early development of widespread metastases. However, it is precisely this aggressiveness to render SCLC one of the most chemosensitive types of solid tumor. Despite most patients have achieved responses with first-line chemotherapy, they relapse within a year of treatment (1). Unfortunately, at relapse, SCLC patients have a very poor prognosis due to drug resistance with a median overall survival (OS) of 2–3 months for patients who do not receive second-line therapy, and rarely more than 6 months for those who receive further therapy (2).
The response to first-line chemotherapy and its duration, the main factors in predicting the efficacy of salvage chemotherapy, has led to classify relapsed SCLC patients into two main groups: sensitive and refractory relapse patients. The ‘sensitive relapse’ patients are those who respond to initial chemotherapy and relapse after more than 60–90 days after the end of chemotherapy; the ‘refractory relapse’ patients are those whose tumor is stable or progresses during the initial chemotherapy or who have a recurrence within 60–90 days after the end of chemotherapy. A meta-analysis collecting the data from six trials involving intravenous topotecan-based second-line chemotherapy was performed to validate these criteria, which were based on small old studies (3-5), and also to assess potential additional clinical parameters predictive of objective response rate (ORR) and OS. This study confirmed that treatment-free interval (TFI) <60 days is the cut-off to consider SCLC patients as refractory to second-line chemotherapy and with poor OS. Moreover, patients with liver metastasis and/or performance status (PS) 2 and/or low albumin, regardless of TFI, have similarly poor outcome (6).
There are no optimal drugs for the treatment of recurrent SCLC but only agents registered for this use such as topotecan, which remains the standard-of-care for the treatment of second-line platinum-sensitive SCLC patients worldwide, and amrubicin which is registered only in Japan (7).
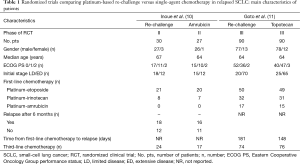
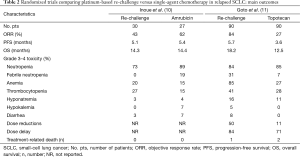
The need to differ sensitive from refractory SCLC patients is very important because a longer drug holiday is a major reason for causing tumors to become resensitized to the same therapy, typically with the same dose and regimen. A possible explanation for SCLCs responding to drug re-challenge after relapse is that the tumor cells were not resistant when therapy was discontinued (i.e., after the standard six cycles of platinum-based chemotherapy). In fact, platinum-based re-challenge represents a potential strategy for the management of relapsed or progressed platinum-sensitive SCLC. The results of this approach derived from retrospective analyses including few patients (8,9). Thus, there is no worldwide agreement on the use of this strategy. A prospective randomized phase II trial, conducted in Japan, compared amrubicin (40 mg/m2 on days 1–3, every 3 weeks) to re-challenge of a platinum-doublet (platinum-etoposide or carboplatin-irinotecan) in 60 patients with SCLC with a TFI >90 days. In the 57 evaluable patients, the ORR, the primary endpoint, was 67% in the amrubicin arm and 43% in the re-challenge group, with a median progression-free survival (PFS) of 5.4 and 5.1 months, respectively. Grade 3 febrile neutropenia was observed in 19% of patients in the amrubicin group while non-hematological toxicities were generally moderate and no treatment-related death was observed in either group (10) (Tables 1,2).
Full table
Full table
Goto et al. performed the first randomized phase III trial addressing this issue, the JCOG0605 study (11). In this trial, 180 patients with SCLC responding to first-line platinum-based treatment but relapsing at least 90 days after completion of therapy were randomly assigned, in 1:1 ratio, to receive cisplatin-combination chemotherapy or topotecan alone. Chemotherapy consisted of five 2-week courses of cisplatin, at the dose of 25 mg/m2 on days 1 and 8, plus etoposide, at the dose of 60 mg/m2 on days 1–3, plus irinotecan, at the dose of 90 mg/m2 on day 8, with the support of granulocyte colony-stimulating factor (G-CSF) starting from day 9 of the first cycle, except for days in which anticancer drugs were given, unless their white blood cell count was at least 10.0×109 cells/L. This regimen was compared with four cycles of topotecan, at the dose of 1.0 mg/m2 on days 1–5, every 3 weeks. The primary endpoint was OS which was 18.2 months in the combination chemotherapy group versus 12.5 months in the topotecan group [hazard ratio (HR) 0.67; 90% confidence interval (CI), 0.51–0.88; P=0.0079]. PFS was 5.7 months in the combination chemotherapy group and 3.6 months in the topotecan group (HR 0.50; 95% CI, 0.37–0.68; P<0.0001). The ORR was 84% and 27% [risk ratio (RR) 0.32; 95% CI, 0.22–0.46; P<0.0001], respectively. Grade 3 or 4 neutropenia was 83% and 86% with febrile neutropenia in 31% versus 7%, anemia 84% versus 28%, thrombocytopenia 41% and 28%, respectively. Treatment-related deaths were reported in two patients treated in the topotecan arm and in one case in the combination chemotherapy arm. Dose reductions occurred in 50% of patients in the combination chemotherapy arm and 11% of patients in the topotecan arm with a dose delay reported in 84% and 71%, respectively. A high percentage of patients received third- and fourth-line chemotherapy in both arms (third-line: 82% in the platinum-based group and 84% in the topotecan group; fourth-line: 46% and 53%, respectively) (11) (Tables 1,2). The results of this trial led the authors to state that the combination of cisplatin, etoposide, and irinotecan could become the standard treatment for selected patients with sensitive relapsed SCLC.
The platinum-based schedule and doses used in this trial derived from previous phase I and II studies (12,13). In fact, in the phase II trial, 40 SCLC patients, who relapsed more than 8 weeks after the completion of first-line therapy, showed an ORR of 78% with a median OS of 11.8 months, the estimated 1-year survival rate was 49%. Grade 3 or 4 neutropenia, and thrombocytopenia were observed in 73% and 33% of the patients, respectively (13). Despite in this phase II study, when compared with the JCOG0605 trial, a similar ORR (78% versus 84%, respectively) with almost similar eligibility patients criteria was reported, a shorter median OS was observed (11.8 versus 18.2 months, respectively). Moreover, in the meta-analysis including 631 relapsed SCLC patients, most of whom were classified as sensitive, the median OS showed by topotecan, given at the intravenous dose of 1.5 mg/m2 days 1–5 (higher than the dose used in the JCOG0605 trial), was 6.3 months (6). Considering these results, the JCOG0605 trial showed a longer median OS for both arms. The possible explanations are that in this randomized phase III trial there was a higher selection of enrolled patients. In fact, they were younger, median age of 64 years, healthier, 97% of patients were PS 0–1, with a longer first remission, 181 and 148 days in platinum-based and topotecan arms, respectively, a high percentage of patients receiving third- and fourth-line therapy in both arms, suggesting an important accrual bias. The slow accrual, about 3.0 patients per month, further underlines that the patients randomized in this trial were highly selected and thus not representing the standard SCLC population. Looking at the characteristics of patients enrolled in the two arms, further imbalances can be noticed: patients receiving the platinum-based regimen had a better PS than those treated with topotecan (PS 0: 58% versus 44%, respectively); median duration of initial response to first-line platinum-based chemotherapy was better in the combination therapy group (181 versus 148 days, respectively). Both are well documented prognostic factors (14). In fact, in case of a late relapse (relapse ≥6 months), the initial first-line therapy, which proved effective, should have been applied again (15). The toxicity reported in the combination arm raises serious concerns about the tolerability of this regimen. In this context, quality of life measurements could have provided evaluable information but, unfortunately, it was not analyzed. Considering the poor prognosis of relapsed SCLC patients, such trials should always include appropriate quality of life measurements to understand the impact of these therapies on daily activities in order to let patients live their lives without undergoing serious toxic effects.
The role of irinotecan in the treatment of SCLC patients seems to be affected by ethnicity. In fact, it is well known that pharmacogenomic differences exist between Caucasian and Asiatic populations, and particularly differences in polymorphisms of UDP-glucuronosyltransferase (UGT1A1), an enzyme that metabolizes irinotecan (16). This could explain the different results in chemosensitivity, compliance and toxicity reported among trials performed in SCLC with different ethnicity (17,18).
Overall, all these reflections have led to consider the combination of cisplatin, etoposide, and irinotecan as the standard treatment only for highly selected Asiatic patients with “very” sensitive relapsed SCLC. Moreover, the significance of the JCOG0605 trial is that it should be the starter for further large randomized phase III trials to confirm better the role of this combination and to define the role of re-challenge therapy in relapsed SCLC patients.
In view of the genetic heterogeneity and the aggressive nature of SCLC, understanding its biology could lead to discover genomic alterations potentially druggable for the development of therapeutic agents. Immunotherapy is a new frontier for the management of cancer, including SCLC. Early trials of various immuno-oncology agents targeting immune checkpoint pathways have shown promising results in these patients. Phase III trials with immune checkpoint agents are underway in any lines of SCLC treatment (7).
In the future, it is of paramount importance to study refractory and sensitive SCLC patients separately within specifically addressed trials, because even if both groups are characterized by a bad prognosis, an even worse one characterizes the refractory patients. Moreover, sensitive relapsed SCLC patients should be studied within trials in which they are stratified based on TFI duration or in trials designed specifically according to TFI duration (i.e., re-challenge approach in presence of TFI ≥6 months). There are many expectations by the drugs under investigation but it is important also to design study with well-defined strategical approach and the hope is that all these expectations are met to offer new and improved treatment options for SCLC patients.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Ming-hui Zhang (Department of Medical Oncology, Harbin Medical University Cancer Hospital, Harbin, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Jackman DM, Johnson BE. Small-cell lung cancer. Lancet 2005;366:1385-96. [Crossref] [PubMed]
- Rossi A, Martelli O, Di Maio M. Treatment of patients with small-cell lung cancer: from meta-analyses to clinical practice. Cancer Treat Rev 2013;39:498-506. [Crossref] [PubMed]
- Giaccone G, Donadio M, Bonardi G, et al. Teniposide in the treatment of small-cell lung cancer: the influence of prior chemotherapy. J Clin Oncol 1988;6:1264-70. [PubMed]
- Ebi N, Kubota K, Nishiwaki Y, et al. Second-line chemotherapy for relapsed small cell lung cancer. Jpn J Clin Oncol 1997;27:166-9. [Crossref] [PubMed]
- Onoda S, Masuda N, Seto T, et al. Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol 2006;24:5448-53. [Crossref] [PubMed]
- Ardizzoni A, Tiseo M, Boni L, et al. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur J Cancer 2014;50:2211-8. [Crossref] [PubMed]
- Rossi A, Sacco PC, Sgambato A, et al. Optimal drugs for second-line treatment of patients with small-cell lung cancer. Expert Opin Pharmacother 2016;17:969-76. [Crossref] [PubMed]
- Garassino MC, Torri V, Michetti G, et al. Outcomes of small-cell lung cancer patients treated with second-line chemotherapy: a multi-institutional retrospective analysis. Lung Cancer 2011;72:378-83. [Crossref] [PubMed]
- Genestreti G, Tiseo M, Kenmotsu H, et al. Outcomes of Platinum-Sensitive Small-Cell Lung Cancer Patients Treated With Platinum/Etoposide Rechallenge: A Multi-Institutional Retrospective Analysis. Clin Lung Cancer 2015;16:e223-8. [Crossref] [PubMed]
- Inoue A, Sugawara S, Maemondo M, et al. Randomized phase II trial comparing amrubicin with re-challenge of platinum doublet in patients with sensitive-relapsed small-cell lung cancer: North Japan Lung Cancer Study Group trial 0702. Lung Cancer 2015;89:61-5. [Crossref] [PubMed]
- Goto K, Ohe Y, Shibata T, et al. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2016;17:1147-57. [Crossref] [PubMed]
- Sekine I, Nishiwaki Y, Kakinuma R, et al. Phase I/II trial of weekly cisplatin, etoposide, and irinotecan chemotherapy for metastatic lung cancer: JCOG 9507. Br J Cancer 2003;88:808-13. [Crossref] [PubMed]
- Goto K, Sekine I, Nishiwaki Y, et al. Multi-institutional phase II trial of irinotecan, cisplatin, and etoposide for sensitive relapsed small-cell lung cancer. Br J Cancer 2004;91:659-65. [PubMed]
- Kalemkerian GP. Combination chemotherapy for relapsed small-cell lung cancer. Lancet Oncol 2016;17:1033-5. [Crossref] [PubMed]
- Jett JR, Schild SE, Kesler KA, et al. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e400S-19S.
- Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci U S A 1998;95:8170-4. [Crossref] [PubMed]
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85-91. [Crossref] [PubMed]
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43. [Crossref] [PubMed]


