Radiotherapy for a second primary lung cancer arising post-pneumonectomy: planning considerations and clinical outcomes
Introduction
Second primary non-small cell lung cancers (SPLC) are a major cause of death in patients surviving their initial lung cancer (1). Following surgery, the risk of developing a SPLC is five times that of the general population, with a 6-year actuarial risk as high as 20% (2,3). The treatment of SPLC arising post-pneumonectomy is seldom considered, as subsequent surgery is rarely feasible (4-7) and historic outcomes with conventional radiotherapy have been poor with a narrow therapeutic ratio (8,9).
Technological improvements in radiotherapy have enabled higher biologically effective doses to be delivered without increasing toxicity (10). Stereotactic ablative radiotherapy (SABR) is an example of this, where individually tailored multiple non-coplanar fixed beams or rotational arcs are accurately delivered using image guidance to ensure the risk of toxicity in radiosensitive organs is minimized. The use of SABR has enabled an increasing proportion of elderly lung cancer patients to be treated curatively, improving population-based survival (11,12). Using similar techniques, more fractionated radiotherapy (hypofractionated radiotherapy, HFR) has also shown promise (13).
In 2009, we reported our initial experience using SABR to treat 15 patients with early-stage SPLC arising post-pneumonectomy (14). After a short follow-up, the actuarial 1-year survival was 92%. Here, we report clinical outcomes including late toxicity after long-term follow-up in a larger cohort treated using modern radiotherapy techniques, including SABR, HFR and conventional radiotherapy (RT). In addition, we specifically evaluated radiotherapy planning considerations in the post-pneumonectomy setting.
Materials and methods
Between April 2003 and December 2011, details of all lung cancer patients referred to the VU University Medical Center were entered into a database documenting patient and tumor characteristics, radiotherapy details and follow-up findings. Patients treated with curative radiotherapy for a SPLC arising post-pneumonectomy were identified. Medical ethics review was not sought, as retrospective reviews fall outside the scope of the Medical Research Involving Human Subjects Act, The Netherlands. For all patients, curative treatment was recommended after multi-disciplinary tumor board review of the case.
As per departmental protocol, curative treatment was considered for all patients that were WHO performance status 0-2, and had no distant metastasis defined on whole-body positron emission tomography and brain magnetic resonance. SABR was the preferred treatment for peripherally located stage I SPLC. In patients with clinical stage II-III disease, and in those with central stage I SPLC where SABR organ at risk constraints could not be met, the recommended treatment was HFR or RT. The organ at risk constraints utilized were consistent with previously published recommendations (15,16). All patients had a planning 4-dimensional CT (4DCT) scan to define the envelope volume within which the gross tumor moved during respiration (17). This was considered the internal target volume. An additional margin of 3-5 mm and 5-10 mm was added to this to create the planning target volume (PTV) for SABR and HFR (or RT) treatments, respectively.
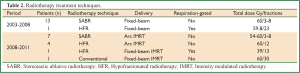
SABR was prescribed to the 80% isodose, ensuring that more than 95% of the PTV received the prescribed dose (18). SABR prescriptions were ‘risk-adapted’, with three fractions of 18-20 Gy delivered for T1 tumors, five fractions of 11-12 Gy for T2 tumors or T1 tumors with broad chest wall contact and eight fractions of 7.5 Gy for central tumors adjacent to the heart, hilus or mediastinum (19). The 11 and 18 Gy fractionation schemes are currently used as modern more accurate dose calculation algorithms suggest this is equivalent to 12 and 20 Gy respectively. HFR and RT treatments were prescribed to an isodose, which ensured more than 95% of the PTV received more than 95% of the prescribed dose (20). The HFR schedule utilized was at the discretion of the treating clinician and constraints accounted for the biological effect of hypofractionation, relative to RT (15,21). Respiration-gated delivery was performed for selected patients undergoing HFR (22). Set-up accuracy was ensured by online image guidance for all patients. For SABR treatments, image guidance prior to 2008 was with bony registration of the thoracic vertebra using orthogonal kV X-ray images (14). Since then, soft-tissue registration of the tumor using cone-beam CT (CBCT) has been used (23). For HFR or RT image guidance was achieved using daily CBCT or orthogonal kV X-ray images with intermittent CBCT. Since 2008, all treatments were delivered using fixed-beam or arc-based intensity-modulated radiotherapy (18,20).
Follow-up included contrast-enhanced CT scans of the lower neck, thorax and upper abdomen at 3, 6 and 12 months, followed by yearly intervals thereafter. Recurrences were defined as: local (LR) with failure in or adjacent to the PTV, regional (RR) with failure in ipsilateral hilus, mediastinum or supra-clavicular fossa and distant (DR) with failure at other sites. Toxicities were scored using the common toxicity criteria for adverse events (CTCAE, v4.0). Three thoracic radiation oncologists (CJH, FJL and PDH) reviewed the clinical records and verified all CTCAE grade 3 or higher toxicities.
Statistical considerations
Descriptive statistics were used for patient, tumor and treatment characteristics. The timing of events was calculated from the first day of radiotherapy. Actuarial survival and recurrence outcomes were estimated using the Kaplan-Meier method. Median follow-up was determined using the reverse Kaplan-Meier method. Lung dose-volume histogram (DVH) statistics were calculated using the total lung minus the PTV (Lung-PTV). These included the mean lung dose (MLD), and the V5, V10, V20 and V30, where the V5 is the Lung-PTV percentage volume receiving 5 Gy or higher. All statistical analyses were two-sided with P≤0.05 threshold for statistical significance and performed using Statistical Package for Social Sciences (version 18.0).
Results
Patient and tumor characteristics
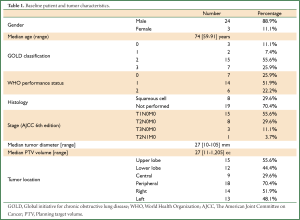
Twenty-seven patients underwent curative radiotherapy for a SPLC arising post-pneumonectomy. Table 1 summarizes the baseline patient characteristics. The median patient age was 74 years (range, 59-91 years). The majority of patients (78%, 21/27) were WHO performance status 0-1 and the remainder performance status 2. Five patients had previously received adjuvant post-operative RT to total doses of between 45-67.5 Gy. The median time between pneumonectomy and SPLC was 91 months (range, 5-343 months), with 89% (24/27) occurring more than two years later. All patients fulfilled the criteria defined by Martini et al. for differentiating recurrence from SPLC (24). Histological confirmation was obtained in 30% (8/27) of patients and a clinical diagnosis made in the remainder on the basis of a new and/or growing lesion with malignant CT features and/or local PET uptake (25,26). The median tumor diameter was 27 mm (range, 10-105 mm) and PTV volume was 27 cc (range, 11-1,205 cc). The median overall follow-up was 52 months (95% CI, 37-67 months). Table 2 summarizes how various radiotherapy techniques were used in treating these 27 patients.
Full table
Full table
Clinical outcomes
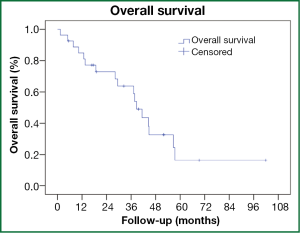
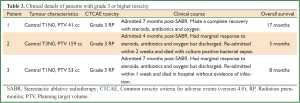
The median overall survival was 39 months (95% CI, 33-44 months, Figure 1). Actuarial 3-year survival was 63% (95% CI, 43-84%). Any recurrence developed in four (15%) patients. Actuarial 3-year rates of LR, RR and DR were 8% (95% CI, 0-21%), 10% (95% CI, 0-23%) and 9% (95% CI, 0-20%), respectively. The patterns of recurrence were isolated LR, isolated RR, isolated DR and RR with DR occurring after a median time of 6 months (95% CI, 0-17 months) post-radiotherapy. Three (11%) patients developed grade 3 or higher radiation pneumonitis (RP), including one in whom RP likely contributed to his premature death (grade 5). None of these patients had received previous radiotherapy. As detailed in Table 3, these patients were all admitted 4-7 months post-treatment with symptoms, including fever, non-productive cough and increasing shortness of breath. There were no non-RP grade 3 or higher toxicities observed.
Full table
Planning considerations
Of the four patients receiving HFR in 12 fractions, 75% (3/4) developed grade 3 or higher pneumonitis. These patients had a median PTV volume 89 cc (range, 41-159 cc) and all had centrally located tumors. The three patients receiving RT or HFR in 13 or more fractions did not develop grade 3 or higher RP despite having larger tumors (217, 400 and 1,205 cc), that were also centrally located. The lung-PTV DVH metrics for these patients were worse than in those receiving HFR in 12 fractions, Table 4. Patients receiving HFR in 12 fractions all had radiotherapy plans, where central organ at risk constraints were prioritized over lung constraints. In addition, full arcs without avoidance sectors were used to deliver treatment. In none of the patients receiving RT or HFR in 13 or more fractions was treatment delivered using arcs and in two respiratory gating was used.
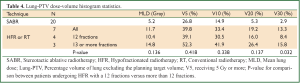
Full table
Discussion
Even with modern surgical techniques, pneumonectomy still constitutes approximately 10% of curative resections (27). Despite SPLC being a significant cause of death amongst lung cancer survivors (1), reporting the use of surgery post-pneumonectomy has been uncommon and included small patient numbers, suggesting it is seldom feasible (4-7). Utilizing modern radiotherapy techniques, we found a median survival of 39 months (95% CI, 33-44) could be achieved in this setting. The 3-year actuarial risk of LR and RR was 8% and 10% respectively, no different from that reported after SABR for early stage tumors (28).
After a median follow-up of 52 months, RP was the only grade 3 or higher toxicity and was observed in 11% (3/27) of patients. A number of radiotherapy planning considerations influence risk of RP in the post-pneumonectomy setting. When using SABR, we previously found the volume of lung receiving 5 Gy or more best predicted the risk of high-grade RP (29). For patients receiving 12 fractions, treatment was delivered using full arcs without avoidance sectors, thereby increasing low dose lung exposure. In the patients treated with 13 or more fractions, treatment was delivered using multiple static beams, which resulted in areas of lung that were spared, as would have been the case when using avoidance sectors. Additionally, with SABR the potential risks of central organ toxicity (30), has led to the highest priority being given to reducing mediastinal doses, an approach we applied to those receiving HFR in 12 fractions. This was however not required given our recent report using SABR in eight fractions where grade 3 toxicity was observed in 6% (4/63) of central tumors treated (31). Patients receiving HFR in 13 or more fractions also had gated treatment delivery, a fact that likely contributed to why no toxicity was observed in these patients. Currently when treating SPLC arising post-pneumonectomy we carefully consider the fractionation schedule, balance the risks of central organ toxicity against RP and utilize less conformal plans with avoidance sectors.
Following pneumonectomy, subsequent surgery is typically limited to either segmental or wedge resections (4-7), however limited resections such as these appear to achieve inferior local control compared to SABR (32), which was the predominant (20/27) radiotherapy technique used in this study. Recently, a randomized trial testing local control after limited resection, with and without intra-operative radiotherapy in high-risk surgical patients was closed. In this study, the rate of grade 3 or higher toxicity at 90-days was 37% in the combined treatment arm, a finding associated with respiratory function and likely to be higher in single lung patients (33). RT is similarly feasible post-pneumonectomy (9), however the available evidence suggests that this is inferior to SABR (34). Case reports have described radiofrequency ablation and percutaneous cryoablation for SPLC and metastasis occurring post-pneumonectomy (35,36). Prospective data confirming the long-term efficacy of these treatments are however lacking (37). Additionally, pneumothorax requiring chest tube occurs in 15% of patients with two lungs, which is again likely to be worse in single lung patients (37).
A limitation of our study is the fact that a significant majority of patients did not have histologically confirmed SPLC prior to radiotherapy, which was in part due to the risks associated with biopsy in this setting. However, in The Netherlands there is a low incidence of PET-positive benign disease and lesions were assessed using a clinically validated algorithm based on CT and PET findings in the multi-disciplinary setting (25,26,38). The accuracy of this approach is suggested by the patterns of recurrence for pathologically confirmed and unconfirmed lung cancer following SABR, which is different (39).
To the best of our knowledge, this represents the largest series describing clinical outcomes for the curative treatment of SPLC arising post-pneumonectomy. The American Association for Thoracic Surgery recently recommended annual low-dose CT surveillance for those able to tolerate subsequent curative treatment (40). As prolonged survival can be achieved with modern radiotherapy techniques, our findings reiterate the importance of such follow-up and referral for a radiotherapy opinion. Should radiotherapy be offered, the risk of RP should be acknowledged and treatment planned accordingly.
Acknowledgements
Disclosure: The VU University Medical Center has a funded research collaboration with Varian Medical Systems. FJL, WFV, BJS and SUS have received honoraria and travel support from Varian Medical Systems. SAS, CJH and PDH declare no personal conflicts of interest.
References
- Hubbard MO, Fu P, Margevicius S, et al. Five-year survival does not equal cure in non-small cell lung cancer: a Surveillance, Epidemiology, and End Results-based analysis of variables affecting 10- to 18-year survival. J Thorac Cardiovasc Surg 2012;143:1307-13.
- Surapaneni R, Singh P, Rajagopalan K, et al. Stage I lung cancer survivorship: risk of second malignancies and need for individualized care plan. J Thorac Oncol 2012;7:1252-6.
- Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 1998;90:1335-45.
- Kittle CF, Faber LP, Jensik RJ, et al. Pulmonary resection in patients after pneumonectomy. Ann Thorac Surg 1985;40:294-9.
- Westermann CJ, van Swieten HA, Brutel de la Rivière A, et al. Pulmonary resection after pneumonectomy in patients with bronchogenic carcinoma. J Thorac Cardiovasc Surg 1993;106:868-74.
- Spaggiari L, Grunenwald D, Girard P, et al. Cancer resection on the residual lung after pneumonectomy for bronchogenic carcinoma. Ann Thorac Surg 1996;62:1598-602.
- Donington JS, Miller DL, Rowland CC, et al. Subsequent pulmonary resection for bronchogenic carcinoma after pneumonectomy. Ann Thorac Surg 2002;74:154-8; discussion 158-9.
- Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable): a systematic review. Thorax 2001;56:628-38.
- Lagerwaard FJ, Voet PW, van Meerbeeck JP, et al. Curative radiotherapy for a second primary lung cancer arising after pneumonectomy -- techniques and results. Radiother Oncol 2002;62:21-5.
- Haasbeek CJ, Slotman BJ, Senan S. Radiotherapy for lung cancer: clinical impact of recent technical advances. Lung Cancer 2009;64:1-8.
- Haasbeek CJ, Palma D, Visser O, et al. Early-stage lung cancer in elderly patients: a population-based study of changes in treatment patterns and survival in the Netherlands. Ann Oncol 2012;23:2743-7.
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153-9.
- Bogart JA, Hodgson L, Seagren SL, et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol 2010;28:202-6.
- Haasbeek CJ, Lagerwaard FJ, de Jaeger K, et al. Outcomes of stereotactic radiotherapy for a new clinical stage I lung cancer arising postpneumonectomy. Cancer 2009;115:587-94.
- Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys 2011;81:1442-57.
- Hurkmans CW, Cuijpers JP, Lagerwaard FJ, et al. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiat Oncol 2009;4:1.
- Underberg RW, Lagerwaard FJ, Cuijpers JP, et al. Four-dimensional CT scans for treatment planning in stereotactic radiotherapy for stage I lung cancer. Int J Radiat Oncol Biol Phys 2004;60:1283-90.
- Ong CL, Verbakel WF, Cuijpers JP, et al. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010;97:437-42.
- Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685-92.
- Verbakel WF, van Reij E, Ladenius-Lischer I, et al. Clinical application of a novel hybrid intensity-modulated radiotherapy technique for stage III lung cancer and dosimetric comparison with four other techniques. Int J Radiat Oncol Biol Phys 2012;83:e297-303.
- Fowler JF, Tomé WA, Fenwick JD, et al. A challenge to traditional radiation oncology. Int J Radiat Oncol Biol Phys 2004;60:1241-56.
- Spoelstra FO, van Sörnsen de Koste JR, Cuijpers JP, et al. Analysis of reproducibility of respiration-triggered gated radiotherapy for lung tumors. Radiother Oncol 2008;87:59-64.
- Grills IS, Hugo G, Kestin LL, et al. Image-guided radiotherapy via daily online cone-beam CT substantially reduces margin requirements for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:1045-56.
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9.
- van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002;359:1388-93.
- Herder GJ, Kramer H, Hoekstra OS, et al. Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: a Dutch cooperative randomized study. J Clin Oncol 2006;24:1800-6.
- Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg 2005;80:2051-6; discussion 2056.
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9.
- Ong CL, Palma D, Verbakel WF, et al. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): planning considerations and early toxicity. Radiother Oncol 2010;97:431-6.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9.
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011;6:2036-43.
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35.
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Thirty- and ninety-day outcomes after sublobar resection with and without brachytherapy for non-small cell lung cancer: results from a multicenter phase III study. J Thorac Cardiovasc Surg 2011;142:1143-51.
- Palma DA, Senan S. Early-stage non-small cell lung cancer in elderly patients: should stereotactic radiation therapy be the standard of care? Int J Radiat Oncol Biol Phys 2012;84:1058-9.
- Yamauchi Y, Izumi Y, Yashiro H, et al. Percutaneous cryoablation for pulmonary nodules in the residual lung after pneumonectomy: report of two cases. Chest 2011;140:1633-7.
- Ambrogi MC, Fanucchi O, Lencioni R, et al. Pulmonary radiofrequency ablation in a single lung patient. Thorax 2006;61:828-9.
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8.
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490-6.
- Verstegen NE, Lagerwaard FJ, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011;101:250-4.
- Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8.