Late recurrence of basaloid carcinoma initially treated as a small cell lung cancer
Introduction
Basaloid carcinoma (BC) is a rare pulmonary neoplasm of epithelial etiology that mimics small cell lung carcinoma (SCLC), large cell neuroendocrine carcinoma (LCNEC), or adenoid cystic carcinoma (ACC) on histopathology. We present a case of a patient who had a neoplasm originally considered and treated as a SCLC with complete response. The tumor recurred locally 8 years later and was resected. Pathologic evaluation showed a BC. This case raises issues associated with treating BC.
Case
A 55-year-old woman with a history of chronic obstructive pulmonary disease (COPD) and 60 pack-year history of smoking, was admitted to the hospital with right-sided chest pain, worsening dyspnea at rest, and fatigue. Chest CT revealed a 4.8 cm × 4.0 cm mass in the right lower lobe with enlarged mediastinal lymph nodes (Figure 1A). Bronchoscopy revealed a verrucous protrusion at the entrance of the anterolateral segments of the right lower lung. Bronchial biopsies yielded the diagnosis of SCLC (Figure 2A). Metastatic work-up with a brain, abdomen and pelvis CT scan was negative; therefore, she was staged as having a cT2aN2 SCLC.
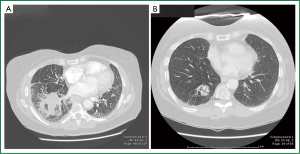
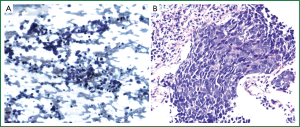
She subsequently underwent concurrent chemoradiation therapy with cisplatin and etoposide and 60 Gray of radiation followed by prophylactic cranial irradiation (PCI). Repeat chest CT showed resolution of her lung mass and lymphadenopathy. Annual chest CTs demonstrated no evidence of disease until 8 years after therapy when a surveillance chest CT revealed a new 2.5 cm × 1.8 cm lesion in the right lower lobe, medial and posterior to the site of her original tumor (Figure 1B). A CT-guided biopsy of the lesion suggested recurrent SCLC (Figure 2B). Metastatic work-up with a PET scan and MRI of the brain was negative again. Following a negative cervical mediastinoscopy, she underwent a right lower lobectomy via a muscle-sparing thoracotomy. She had an unremarkable postoperative course and was discharged on postoperative day 6.
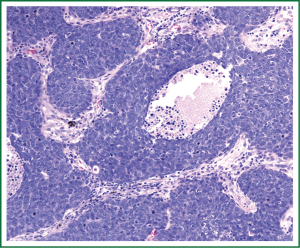
Final pathological diagnosis was BC (Figure 3). The tumor was morphologically identical to her previous tumor. In the initial and recurrent diagnoses, a different pathologist determined the histopathology; however, for the recurrence, both tumors were reviewed and determined to be identical. It was composed of small to intermediate sized cells, with a trabecular growth pattern. The cells had high nuclear to cytoplasmic ratios, nuclear molding, and high mitotic rate. Necrosis was evident. The tumor reacted with the high molecular weight cytokeratin antibody 34βE12. Other neuroendocrine markers including chromogranin, synaptophysin, and CD56 were negative. She subsequently was treated with adjuvant cisplatin and etoposide and has had an unremarkable course. She remains disease free 2 years since her salvage lobectomy.
Discussion
BC of the lung is a rare neoplasm characterized by small, highly mitotic cells that palisade peripherally. They represent approximately 6% of non-small cell lung cancer (NSCLC) (1). They are classified either as a variant of large cell carcinoma in their pure form, or as a basaloid variant of squamous cell carcinoma when squamous differentiation is present (2). It remains unclear whether BC portends a worse prognosis compared to other NSCLCs. Patients with BC have a reported median survival of 22-34 months and a 5-year survival of 10-37% (1,3,4). These figures are primarily from patients who have had lung resections.
BC is difficult to diagnose and may be misdiagnosed as SCLC, ACC, or LCNEC due to similarities in morphology (1,3). Immunohistochemical markers, such as TTF-1 and 34βE12, may be helpful in differentiating between BC from other types of lung carcinomas (5). There are no separate diagnostic criteria for BC from other lung cancers. The definitive diagnosis is made based upon histopathology and immunohistochemistry.
The data to guide treatment of BC is sparse; however, they generally are treated like the more common histological subtypes of NSCLC. Interestingly, the patient presented in this report was treated successfully in a SCLC treatment paradigm with definitive concurrent chemoradiation therapy followed by PCI. Treatment for locally advanced NSCLC is similar (other than PCI), with a slightly different schedule of cisplatin and etoposide. Salvage resection after late recurrence was considered as there were no other obvious sites of disease, clearly an atypical course for a small cell lung cancer. Owing to its rarity, there are no randomized controlled studies assessing the optimal treatment of BC outside of the standard treatment for lung cancer.
It is unclear if the recently identified lesion truly represents a local recurrence, rather than a new primary tumor. The long interval (8 years) between the two lesions argues against a recurrence. In one study with a median follow-up of 26.5 months, 38% of patients with BC had a median recurrence of 10 months after surgery, and of those, only 15% were local (3). Nevertheless, the patient presented in this report had strong evidence supporting the diagnosis of a late local recurrence by virtue of the second lesion occurring in the same location and having the identical histology as the first lesion.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Moro-Sibilot D, Lantuejoul S, Diab S, et al. Lung carcinomas with a basaloid pattern: a study of 90 cases focusing on their poor prognosis. Eur Respir J 2008;31:854-9.
- Brambilla E, Travis WD, Colby TV, et al. The new World Health Organization classification of lung tumours. Eur Respir J 2001;18:1059-68.
- Kim DJ, Kim KD, Shin DH, et al. Basaloid carcinoma of the lung: a really dismal histologic variant? Ann Thorac Surg 2003;76:1833-7.
- Brambilla E, Moro D, Veale D, et al. Basal cell (basaloid) carcinoma of the lung: a new morphologic and phenotypic entity with separate prognostic significance. Hum Pathol 1992;23:993-1003.
- Sturm N, Lantuéjoul S, Laverrière MH, et al. Thyroid transcription factor 1 and cytokeratins 1, 5, 10, 14 (34βE12) expression in basaloid and large-cell neuroendocrine carcinomas of the lung. Hum Pathol 2001;32:918-25.