More options, more considerations: how new treatment options influence clinical decision making
Given the fact that the majority of cases of metastatic breast cancer (MBC) are incurable, the primary goal when treating these patients is to prolong overall survival (OS) and palliate symptoms, but not at the expense of quality of life (QoL). The choice of therapy should be made on an individual, patient-by patient basis, taking into account various prognostic or predictive factors (1). Patients with rapidly progressing disease or symptoms may gain the most from combination or more aggressive chemotherapy; a less toxic approach may suit those with more gradual disease progression. It is not a trivial task to achieve the correct balance between efficacy and toxicity when choosing any of the relatively protracted chemotherapeutic regimens that are currently used for MBC.
Previously, several clinical trials have assessed the efficacy of capecitabine as a first line therapy for MBC, given its high clinical activity and favorable toxicity. Two randomized phase III trials have shown that capecitabine monotherapy is comparable to control treatments with either cyclophosphamide, methotrexate, and 5-FU (CMF) (2), or pegylated liposomal doxorubicin (PLD) (3), both in terms of OS and progression free survival (PFS) (Table 1). In those studies, 80% of patients manifested hormone receptor positive disease (2), with all patients experiencing in excess of 12 months of disease free interval (3). Although the comparators used in those trials might now appear dated, capecitabine monotherapy showed an acceptable clinical efficacy, and a more manageable safety profile. Those data agree with other reports derived from first line trials (4).
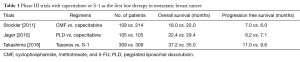
Full table
S-1 is an oral fluorouracil derivative that, like capecitabine, is widely used in Japan. S-1 contains tegafur (a fluorouracil pro-drug), gimeracil, and oteracil. Gimeracil prolongs the half-life of tegafur, while oteracil improves its toxicity profile. In the article that accompanies this commentary, Takashima et al. (5) present data from a phase III trial of S-1 vs. taxanes, when used as a first line therapy for HER2 non-amplified MBC. In contrast to previous studies using capecitabine, the value of this study is its confirmation of the efficacy of an oral fluorouracil agent compared with standard first line therapy using taxanes.
Patients allocated to the taxane group received paclitaxel either weekly, every 3 weeks, or docetaxel every 3 weeks, with these decisions at the discretion of the investigators. The dose of S-1 was 40–60 mg bid for 28 days, followed by a 2-week break. Relevant stratification factors included liver metastasis, previous taxane or 5-FU treatment, and hormone receptor positivity. The primary endpoint was OS, with a pre-specified non-inferiority margin of 1.333 for the hazard ratio (HR). Around 30% of patients had been previously exposed to taxanes, and 10% to oral fluorouracil as an adjuvant. Approximately 75% of patients had hormone receptor positive disease, with 57% having received endocrine therapy in a metastatic setting.
The study findings indicated that S-1 had a significant activity in MBC, with a median OS of 35.0 months (95% CI, 31.1–39.0), which was not inferior to that of the control standard therapy using taxanes (HR =1.05; 95% CI, 0.86–1.27; Pnon-inferiority=0.05). Overall safety profiles were, as expected, substantially more favorable for S-1 treatment, especially in terms of alopecia, edema, and sensory neuropathy. On the other hand, adverse hematological events were comparable between groups. In terms of global health status and functional status, as evaluated by the EORTC QLQ-C30 questionnaire, S-1 treatment was superior compared to the control therapy.
To transition these findings into clinical practice, it is critical that we now identify the patient characteristics that would predict a benefit from S-1 therapy. In subgroup analyses, there were no specific clinical criteria indicative for either treatment. However, while hormone receptor positive patients showed a similar efficacy between the two groups (HR =0.96; 95% CI, 0.72–1.26), patients with triple negative breast cancer tended to benefit more from taxane treatment (HR =1.29; 95% CI, 0.88–1.89). Furthermore, S-1 was less effective for patients with liver metastases compared with therapy using taxanes (HR =1.22; 95% CI, 0.88–1.68), although patients with extensive liver metastases, and symptomatic lymphatic pulmonary metastases, were excluded in this study. Another discussion point was that approximately 20% of patients who had hormone receptor positive disease, without symptomatic problems based on eligibility criteria were included in this trial without prior endocrine therapy. Therefore, we can assume that majority of patients participating in this trial had favorable disease characteristics. Patients with less aggressive disease could therefore benefit from this less toxic treatment, which emphasizes the importance of using individual patient-specific criteria in therapeutic decision-making.
In summary, this study was of value in accessing the efficacy and safety of an oral S-1 treatment compared to the current standard first line regimen using taxanes, and showed comparable clinical outcomes for patients with HER2 non-amplified MBC. These data extend the treatment options for MBC, although the appropriate choice of therapy should still be guided by the biologic characteristics of each individual's disease.
Acknowledgements
None.
Footnote
Provenance: This is an invited Commentary commissioned by Editor-in-Chief Nanshan Zhong (Academician, Chinese Academy of Engineering. Guangzhou Institute of Respiratory Disease, Guangzhou, China).
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Beslija S, Bonneterre J, Burstein H, et al. Second consensus on medical treatment of metastatic breast cancer. Ann Oncol 2007;18:215-25. [Crossref] [PubMed]
- Stockler MR, Harvey VJ, Francis PA, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol 2011;29:4498-504. [Crossref] [PubMed]
- Jäger E, Al-Batran S, Saupe S, et al. A randomized phase III study evaluating pegylated liposomal doxorubicin (PLD) versus capecitabine (CAP) as first line therapy for metastatic breast cancer (MBC): Results of the PELICAN study. J Clin Oncol 2010;28:abstr 1022.
- O'Shaughnessy JA, Kaufmann M, Siedentopf F, et al. Capecitabine monotherapy: review of studies in first-line HER-2-negative metastatic breast cancer. Oncologist 2012;17:476-84. [Crossref] [PubMed]
- Takashima T, Mukai H, Hara F, et al. Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 2016;17:90-8. [Crossref] [PubMed]


