Prosthetic valve endocarditis after transcatheter aortic valve implantation-diagnostic and surgical considerations
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as an excellent alternative therapeutic option to surgical aortic valve replacement (SAVR) in high-risk or inoperable patients who are suffering from severe symptomatic aortic valve disease (1). Prosthetic valve endocarditis (PVE) is a life threatening condition. A few cases having successful SAVR after TAVI were reported. TAVI-PVE has in general been treated conservatively due to the inherent high operative risk (2). Thus, there is no established consensus on the management of this patient category (3). The prevalence of endocarditis in patients with a prosthetic valve has ranged from 1% to 6% with an incidence of 0.3% to 1.2% within the first year after intervention (4,5). The causative microorganisms typically are staphylococci, streptococci and enterococci (4).
Case presentation
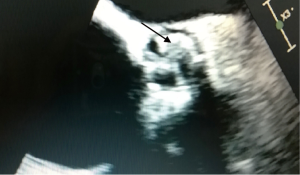
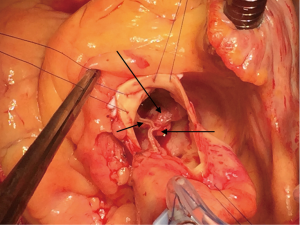
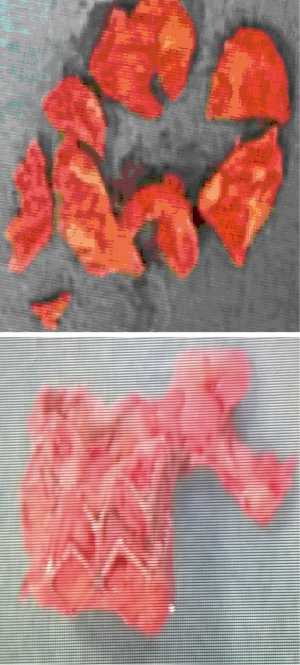
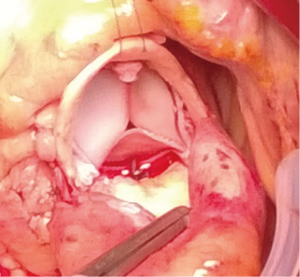
An 80-year-old woman with diabetes mellitus, chronic obstructive lung disease, steroid treated gout arthritis, atrial fibrillation with concomitant anticoagulation treatment, profuse atherosclerosis of the ascending aorta underwent according to the standard local TAVI protocol successfully transfemoral TAVI with an Edwards-Sapien XT 23 mm valve (Figures 1-3). The in-hospital course was uneventful and the patient was discharged home three days after the procedure. At two months routine follow-up the patient reported significantly relief of symptoms, and transthoracic (TTE), transesophageal (TEE) echocardiography, and heart computed tomography (HCT) revealed a normal left ventricular ejection fraction (LVEF), and proper implantation of the transcatheter heart valve (THV) prosthesis without evidence of cusp anatomy or function abnormalities. Three months after the TAVI procedure, the patient received dental treatment. According to local practice, endocarditis prophylactics were not administered. Over the subsequent 3 weeks, the patient developed recurrent fever, abdominal pain, nausea, diarrhoea, and vaginal bleeding. Urine cultures, blood examinations, cystourethroscopy, ultrasound examinations, abdominal CT and endometrial biopsy were normal. TTE and TEE was performed twice without signs of malfunctioning of the THV or signs of PVE. One month later enterococcus faecalis growth sensitive for vancomycin and ampicillin was documented in blood cultures. She was on vancomycin then. A 6-week-long treatment with antibiotics was commenced. Repeated TEE revealed a 6-mm large vegetation on the TAVI prosthesis (Figure 4). A week later the patient became septic, had abdominal pain, respiratory and renal insufficiency, intermittent ventricular tachycardia, temporary convulsions and disorientations. CT revealed large spleen infarction and multiple small cerebral emboli. TEE showed moderate reduced LVEF to 45%, as well as increased size of the vegetations and aortic prosthesis insufficiency (Figures 5-7). Despite her high risk, surgical treatment with replacement of the transcatheter Edwards-Sapien XT device was performed. In Moderate hypothermia the heart was stopped by retrograde cardioplegia. The device was correctly positioned. Vegetations were clearly identified on all three cusps and there was a leak (Figure 8). To avoid damage to the surrounding structures, we excised the three infected leaflets first and then the stent was crumpled and bent and twisted for safe removal (Figure 9). There were no vegetations on the calcified native valve. The annulus was decalcified before implanting a Carpentier-Edwards Perimount 23 mm bioprosthesis (Figure 10). No bacterial DNA was found. Echocardiography revealed a well-positioned aortic valve prosthesis without leakage and normal left ventricular systolic function. She received 6 weeks intravascular vancomycin treatment. At the routine 1, and 3 months follow-up the patient was doing well. Multi-imaging with HCT, TTE and TEE echocardiography demonstrated a well-positioned aortic valve prosthesis (Figure 11).







Discussion
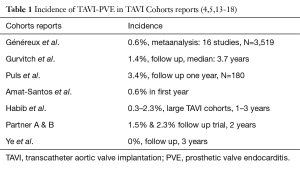
The first successful animal TAVI, which was accomplished at our institution in 1992 led to TAVI application in human in 2002 and TAVI was acknowledge as an alternative treatment to SAVR in high-risk patients with aortic valve stenosis (2). Although more than 800,000 TAVI procedures have been performed worldwide, only a few cases of TAVI-PVE have been reported, with an incidence of 0 to 2.3% of patients enrolled in large TAVI cohorts within the first three years of follow-up (Table 1) (4,16,19). Amat-Santos et al. described the most frequent causal microorganisms as coagulase-negative staphylococci (24%), staphylococcus aureus (21%) and enterococci (21%) and positive vegetation findings in 77% (prosthesis valve leaflets 39%, stent frame 17%, mitral valve 21%). At least one complication such as congestive heart failure, stroke, respiratory insufficiency, pneumonia and sepsis was seen in 87% of PVE patients. The associated in hospital mortality rate was 47% (3). A modified Duke criterion has been used to confirm the diagnosis of TAVI-PVE (20). The predisposing factors mentioned in the reports are advanced age >80 years, high-risk for conventional surgery, multiple comorbidities as diabetes, renal failure, moderate paravalvular aortic regurgitation, suboptimal device position (more than 6 mm into the left ventricular outflow tract) (21), immune-compromised, bacteraemia reactivation, history of myocardial infarction, mitral regurgitation, valve prosthesis and coronary artery bypass grafting. The blood cultures can be positive for enterococcus faecalis, streptococcus, Escherichia coli, and methicillin-resistant staphylococcus aureus (MRSA). PVE has been observed in both transfemoral and transapical TAVI. Access type, procedure-, fluoroscopy length, and contrast amount have no effect on development of PVE (20) The lack of antibiotic prophylaxis during surgical procedures, dental treatment, and coloscopy is discussed as a predisposition for PVE development (19,22-24). PVE may have an atypical and variable clinical presentation which can delay TAVI-PVE diagnosis. Symptoms resembling the respiratory-, urinary-, and gastro-intestinal tract infections often make significant delay in prompt diagnosis and treatment. The echocardiography criteria for the diagnosis of infective endocarditis are not easily applicable in TAVI-PVE as it is hard to show any vegetations. The findings of smaller vegetations, and abscesses are challenging due to the shadowing effect of the prosthetic material, reflections, native valve extensive calcifications and vegetation in the free space between TAVI and calcified native valve would be difficult to detect (20). In advanced stages of PVE, a persisting paravalvular aortic regurgitation, vegetations on one or all prosthetic cusps, aortic root abscess and pseudoaneurysms can be detected. PVE often involves the junction of the sewing ring rather than the leaflets in contrast to our findings. A delay in diagnosis may cause significant morbidity and mortality due to cerebral embolisation, acute renal failure, sepsis and congestive heart failure. TAVI-PVE has in general been treated conservatively due to the inherent high operative risk (19,21). In Puls et al. cohort study PVE mortality was reported about 40% with conservative treatment (20). Surgery in these high risk or inoperable patients in general is considered in case of thromboembolic event, abscess, aneurysm, vegetations, uncontrolled infection and heart failure. Surgical replacement of TAVI has been reported in only four patients with zero 30 days mortality (5,19,22,23,25).
Conclusions
The current paper demonstrated a successful SAVR for TAVI-PVE in a high risk patient with variable and atypical PVE clinical presentation. Further research, echocardiography experience, vigilance for PVE after TAVI, antibiotic prophylaxis for dental and other procedures, and treatment in high specialised departments are re-commended. TAVI-PVE diagnosis is difficult and delays treatment.
Acknowledgements
The authors thank M. Hussain Jalil Ahmad for helping in reconstruction of the videos.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 2009;30:2369-413. [Crossref] [PubMed]
- Olsen NT, De Backer O, Thyregod HG, et al. Prosthetic valve endocarditis after transcatheter aortic valve implantation. Circ Cardiovasc Interv 2015.8. [PubMed]
- Liu XB, Wang JA. Update of transcatheter valve treatment. J Zhejiang Univ Sci B 2013;14:670-5. [Crossref] [PubMed]
- Eisen A, Shapira Y, Sagie A, et al. Infective endocarditis in the transcatheter aortic valve replacement era: comprehensive review of a rare complication. Clin Cardiol 2012;35:E1-5. [Crossref] [PubMed]
- Amat-Santos IJ, Messika-Zeitoun D, Eltchaninoff H, et al. Infective endocarditis after transcatheter aortic valve implantation: results from a large multicenter registry. Circulation 2015;131:1566-74. [Crossref] [PubMed]
- Ahmad K, Klaaborg KE, Hjortdal V, et al. 3-D cardiac CT reveals profuse atherosclerosis of the ascending aorta prior to transcatheter aortic valve implantation. Asvide 2016;3:419. Available online: http://www.asvide.com/articles/1191
- Ahmad K, Klaaborg KE, Hjortdal V, et al. HCT demonstrates proper implantation of Edwards-Sapien XT Transcatheter heart valve prosthesis. Asvide 2016;3:420. Available online: http://www.asvide.com/articles/1192
- Ahmad K, Klaaborg KE, Hjortdal V, et al. 3-D cardiac CT shows profuse atherosclerosis of the ascending aorta and properly implanted transcatheter heart valve prosthesis without anatomical abnormalities. Asvide 2016;3:421. Available online: http://www.asvide.com/articles/1193
- Ahmad K, Klaaborg KE, Hjortdal V, et al. HCT shows well positioned transcatheter valve prosthesis, and prosthetic valve vegetations. Asvide 2016;3:422. Available online: http://www.asvide.com/articles/1194
- Ahmad K, Klaaborg KE, Hjortdal V, et al. TEE demonstrates moderate reduced LVEF, vegetations and aortic prosthesis insufficiency. Asvide 2016;3:423. Available online: http://www.asvide.com/articles/1195
- Ahmad K, Klaaborg KE, Hjortdal V, et al. TEE demonstrates increased size of the vegetations on the transcatheter aortic valve prosthesis. Asvide 2016;3:424. Available online: http://www.asvide.com/articles/1196
- Ahmad K, Klaaborg KE, Hjortdal V, et al. Post SAVR TEE demonstrates well positioned aortic valve bioprosthesis without any sign of leaflets vegetations or insufficiency. Asvide 2016;3:425. Available online: http://www.asvide.com/articles/1197
- Généreux P, Head SJ, Van Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol 2012;59:2317-26. [Crossref] [PubMed]
- Gurvitch R, Wood DA, Tay EL, et al. Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation 2010;122:1319-27. [Crossref] [PubMed]
- Puls M, Eiffert H, Hünlich M, et al. Prosthetic valve endocarditis after transcatheter aortic valve implantation: the incidence in a single-centre cohort and reflections on clinical, echocardiographic and prognostic features. EuroIntervention 2013;8:1407-18. [Crossref] [PubMed]
- Gotzmann M, Mügge A. Fatal prosthetic valve endocarditis of the CoreValve ReValving System. Clin Res Cardiol 2011;100:715-7. [Crossref] [PubMed]
- Eisen A, Shapira Y, Sagie A, et al. Infective endocarditis in the transcatheter aortic valve replacement era: comprehensive review of a rare complication. Clin Cardiol 2012;35:E1-5. [Crossref] [PubMed]
- Gotzmann M, Mügge A. Fatal prosthetic valve endocarditis of the CoreValve ReValving System. Clin Res Cardiol 2011;100:715-7. [Crossref] [PubMed]
- Castiglioni A, Pozzoli A, Maisano F, et al. Endocarditis after transfemoral aortic valve implantation in a patient with Osler-Weber-Rendu syndrome. Interact Cardiovasc Thorac Surg 2012;15:553-4. [Crossref] [PubMed]
- Wong DR, Boone RH, Thompson CR, et al. Mitral valve injury late after transcatheter aortic valve implantation. J Thorac Cardiovasc Surg 2009;137:1547-9. [Crossref] [PubMed]
- Zanuttini D, Armellini I, Bisceglia T, et al. Transcatheter aortic valve implantation for degenerative aortic valve regurgitation long after heart transplantation. Ann Thorac Surg 2013;96:1864-6. [Crossref] [PubMed]
- García-Pardo H, Revilla A, Sevilla T, et al. Staphylococcus aureus endocarditis on transcatheter aortic valves. Rev Esp Cardiol (Engl Ed) 2012;65:771-3. [Crossref] [PubMed]
- Rafiq I, Parthasarathy H, Tremlett C, et al. Infective endocarditis caused by Moraxella nonliquefaciens in a percutaneous aortic valve replacement. Cardiovasc Revasc Med 2011;12:184-6. [Crossref] [PubMed]
- Head SJ, Dewey TM, Mack MJ. Fungal endocarditis after transfemoral aortic valve implantation. Catheter Cardiovasc Interv 2011;78:1017-9. [Crossref] [PubMed]
- Comoglio C, Boffini M, El Qarra S, et al. Aortic valve replacement and mitral valve repair as treatment of complications after percutaneous core valve implantation. J Thorac Cardiovasc Surg 2009;138:1025-7. [Crossref] [PubMed]