Emerging strategies for the treatment of advanced small cell lung cancer
While treatment strategies for non-small cell lung cancer (NSCLC) has made substantial progress in the past two decades with the advent of molecular targeted agents and immune checkpoint inhibitors, advances in the field of small cell lung cancer (SCLC) have been relatively dismal. The mainstay of treatment for advanced SCLC remains platinum-based doublet chemotherapy in the first line setting, with rechallenge of chemotherapy during relapse for platinum-sensitive disease. In the platinum resistant or refractory settings, there are no standard guidelines for treatment, with various phase II studies showing similar response rates (RR) and survival outcomes for different single agent chemotherapies, including topotecan, irinotecan, taxanes and gemcitabine (1).
Multiple molecularly targeted agents have been assessed in SCLC with limited success in improving patient outcomes beyond platinum doublet chemotherapy. One of the earliest mechanisms targeted in SCLC was angiogenesis since high vascular endothelial growth factor (VEGF) levels were found to be a poor prognostic marker for SCLC, and preclinical studies supported the use of anti-VEGF agents to improve tumor responses (2). However, subsequent phase II/III trials undertaken with different anti-VEGF antibodies or small molecule inhibitors were negative, resulting in the discontinuation of anti-VEGF clinical development in SCLC (3). Other signaling pathways have also been assessed in SCLC, including targeted agents against the phosphatidylinositol 3-kinase (PI3K) pathway and critical apoptotic substrates, such as BCL2. Despite strong evidence of antitumor activity in pre-clinical studies, phase II trials have nevertheless been negative (Figure 1) (4).
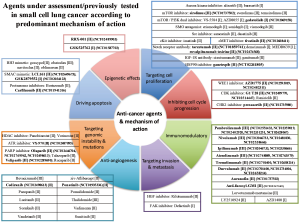
George and co-workers assessed whole genome sequencing data from 110 SCLC primary tumors, which found that TP53 and RB1 loss were almost universal (100% and 93%, respectively) (5). However, TP53 and RB1 are both notoriously challenging to target and are currently not actionable. Interestingly, inactivating mutations in the Notch family were noted in approximately 25% of SCLC tumors, with Notch activation in LSL-N2ICD mice leading to tumor reduction. The Notch signalling pathway has been shown to be important for the control of neuroendocrine differentiation, and its inhibition may be mediated by DLL3 through ASCL1 (6).
Another promising target, poly(ADP-ribose) polymerase (PARP), has been shown in proteomic profiling studies to be dysregulated in SCLC (7). There has already been much success in exploiting the concept of synthetic lethality through the inhibition of PARP in BRCA1/2 mutated ovarian and breast cancers (8,9) The use of PARP inhibitors in SCLC is currently being investigated in the maintenance setting for patients who have responded to first line chemotherapy (ISRCTN73164486), and also in the first line setting in combination with cisplatin/etoposide (NCT01642251).
Molecular analysis has also found that genomic signatures of SCLC are similar to those associated with tobacco exposure (10), in keeping with the fact that SCLC is almost universally associated with smoking. In addition, studies characterising the genomic landscape of different cancers have placed SCLC among those with the highest mutational load, with a non-synonymous mutation rate of 5.5 to 7.4/Mb (11). Importantly, there is increasing evidence that mutational load is a predictor of response to novel immunotherapeutic agents. Subsequent clinical studies involving immune checkpoint inhibitors including anti-cytotoxic T-lymphocyte associated protein 4 (CTLA4) and anti-programmed cell death 1 (PD-1) therapies have shown promising efficacy. Ipilimumab in combination with carboplatin/paclitaxel in the first line setting for extensive stage (ES) SCLC has shown that phased ipilimumab improved immune-related progression-free survival (irPFS) compared to chemotherapy alone (hazard ratio =0.64, P=0.03) (12). In the subgroup of patients with SCLC in the KEYNOTE-028 study treated with pembrolizumab, patients with programmed cell death-ligand-1 (PD-L1) positive tumors had RR of up to 35%, indicating that RR with immunotherapy could be improved with better patient selection criteria (13).
In addition, evidence has suggested that the dual blockade of PD-1 and CTLA-4 pathways may have synergistic outcomes due to the inhibition of non-redundant mechanisms in immune suppression (14,15). Most recently, the CHECKMATE-032 study showed that combination therapy with nivolumab and ipilimumab is more effective than nivolumab monotherapy, with a RR of 23%, median PFS (mPFS) of 3.4 months and 1-year overall survival (OS) of 43% (16). More importantly, survival curves suggest that there could be a long tail, indicating that some patients are experiencing durable responses; it will be interesting to see if these promising data persist with longer follow up. A phase III study is currently investigating the utility of combined immune checkpoint inhibition as maintenance therapy in patients who achieved at least stable disease after four cycles of standard first line platinum doublet chemotherapy (NCT02538666).
In the article published by Gapanova and colleagues in Clinical Cancer Research (17), the authors report that a novel inhibitor-drug conjugate, STA-8866, produced impressive preclinical results in terms of tumor shrinkage and survival in SCLC xenograft and patient-derived xenograft (PDX) models. STA-8666 is a tripartite molecule where a heat shock protein-90 (HSP90)-targeting moiety is conjugated via a cleavable carbamate linker to SN38, the metabolite of irinotecan. The rationale for drug efficacy is based on the understanding that HSP90 is highly expressed in tumor cells compared to normal tissue, thus allowing STA-8666 to deliver drug at higher concentrations to the tumor.
In this study, xenograft and PDX models of SCLC were treated with single agent STA-8666 at varying doses, combination treatment with STA-8666 plus carboplatin, and other cytotoxic regimens including irinotecan, topotecan, etoposide, carboplatin, ganetespib (a HSP90 inhibitor), and carboplatin/etoposide. The study showed robust activity of STA-8666 both as monotherapy at 150 mg/kg and in combination at 50mg/kg with carboplatin in the NCI-H69 xenograft model, where tumors showed regression below the detectable range in all mice after 3 doses of treatment (6/6 in single agent, 11/11 in combination treatment). Durable responses were observed, with 3/6 mice showing no recurrence with STA-8666 monotherapy over 3 months, while 6/11 mice had no recurrence within 120 days with combination treatment. Importantly, disease recurrence was controlled by repeated administrations of STA-8666, with rapid tumor regression to undetectable levels upon treatment rechallenge.
Pharmacokinetic and pharmacodynamics studies showed that STA-8666 is highly concentrated in tumor tissue both in cleaved and uncleaved form, indicating the potential for continued SN38 release. Immunohistochemistry (IHC) analysis of tumor samples at different timepoints also support the presence of persistent necrosis post treatment. Studies of the downstream effects of STA-8666 demonstrated activity against kinases involved in DNA damage and cell cycle checkpoint involved in G2/M phase arrest, confirming its mechanistic role in causing cell death. Taken together, these results support the fact that STA-8666 is highly effective both as monotherapy and in combination with carboplatin for the treatment of SCLC, and will warrant clinical studies to validate these findings.
The use of irinotecan as a cytotoxic in treatment of SCLC has been well studied, providing good scientific rationale for the use of STA-8666 in this setting, with its drug conjugate SN38, an active metabolite of irinotecan. While several phase II studies support the use of irinotecan for the treatment of SCLC in the relapsed setting (18), its role in the first line setting is somewhat controversial. The initial Japan Clinical Oncology Group (JCOG) study by Noda and co-workers showed superior survival in patients with ES SCLC treated with the combination of cisplatin/irinotecan versus cisplatin/etoposide (P=0.002), with an improvement in RR from 67% to 84% (P=0.02) (19). Nonetheless, three further studies carried out in Western populations in Europe and North America were unable to recapitulate such an improved survival (20-22). The combination of platinum with etoposide has therefore remained the standard of care in the first line setting for ES SCLC. Regardless, irinotecan monotherapy remains a viable treatment option in the setting of relapsed SCLC, with efficacy rates similar to other single agent treatments.
Recently, various other drug conjugates including antibody-drug conjugates (ADCs) such as lorvotuzumab mertansine and rovalpituzumab tesirine have been studied in SCLC. Rovalpituzumab tesirine is a humanised anti-delta-like 3 (DLL3) monoclonal antibody conjugated to pyrrolobenzodiazepine (PDB) dimer toxin that binds to a DNA minor groove resulting in DNA damage (23). It showed a RR of 16% and disease control rate (DCR) of 31% in a phase Ib trial involving patients with relapsed SCLC and large cell neuroendocrine cancer. Looking at the subgroup of patients deemed to be DLL3-positive (tumor DLL3 expression in ≥50% of tumor cells by IHC), the RR and DCR improved to 31% and 85%, respectively (24). More importantly, antitumor responses were seen in both the platinum sensitive and resistant/refractory settings. The drug was generally well tolerated, with thrombocytopenia and serosal effusion being the most common grade 3 adverse events. Another ADC that has been assessed in SCLC is lorvotuzumab mertansine, involving a CD56 binding antibody conjugated to a microtubule inhibitor DM-1. While preclinical and phase I data for lorvotuzumab mertansine appeared promising, the phase II trial investigating its role in combination with carboplatin/etoposide in the first line SCLC setting was discontinued due to a lack of efficacy and possible increased risk of infection and infection-related deaths (25).
While STA-8666 has been shown to be highly efficacious in the pre-clinical setting in the study by Gapanova and colleagues (17), numerous therapies tested in SCLC over the past decade have time and again demonstrated that robust preclinical data do not necessarily translate into clinical success. Apart from the examples already discussed in this article, other targeted therapies such as vismodegib, an inhibitor of the Hedgehog pathway (26), and ABT-263, a BCL2 inhibitor (27), have similarly shown high efficacy in PDX models, but subsequently failed in clinical trials. Strategies to bridge this valley of death between the preclinical and clinical settings are clearly needed urgently.
Besides the inherent differences in mouse models and human subjects, another possible reason for the high drug attrition rates in late stage SCLC clinical studies could be due to the fact that it has been challenging to recapitulate the multitude of genetic aberrations found in human tumors of SCLC in genetically engineered mouse models (GEMMs) (4). For example, the complexity of genetic and epigenetic changes brought about by carcinogens in tobacco smoke may not be reflected in these GEMMs, resulting in the simplification of oncogenic pathways involved and an oversight of possible bypass mechanisms employed by tumors to escape cell death. Nevertheless, in the absence of better animal models, such GEMMs remain the best surrogate for preclinical studies in the current setting.
Many drugs that have been combined with first line therapy with platinum/etoposide treatment have failed to show improved outcomes, likely due to the fact that SCLC is a highly chemosensitive tumor with impressive RR of 70–85% (28), making it challenging to improve such outstanding outcomes without additional significant toxicities. Therefore, rather than combine STA-8666 with chemotherapy in the advanced SCLC setting, STA-8666 should be investigated in a maintenance setting to improve PFS and delay the inevitable relapse seen in patients with SCLC.
Looking to the future, the next steps forward for STA-8666 will be crucial in establishing its niche registration space in an ever-expanding armamentarium of novel trial agents that are being assessed along the SCLC treatment pathway. Ultimately, the challenge now is to determine how best to exploit our improved understanding of the biology of SCLC and the new range of antitumor agents available in the clinic, and to translate them into meaningful management strategies to improve treatment outcomes in this disease of urgent unmet need.
Acknowledgements
Funding: The Drug Development Unit of the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research is supported in part by a programme grant from Cancer Research UK. Support is also provided by the Experimental Cancer Medicine Centre (to The Institute of Cancer Research) and the National Institute for Health Research Biomedical Research Centre (jointly to the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research).
Footnote
Provenance: This is an invited Editorial/Commentary/Perspective commissioned by the Section Editor Heng Yang (Augusta University Cancer center, Georgia, USA).
Conflicts of Interest: TAY has received research support from AstraZeneca and Merck, and has served on Advisory Boards and received travel support from Pfizer and Bristol Myers Squibb. J S Lim is supported by the National Medical Research Council (NMRC) Research Training Fellowship, Singapore.
References
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664-72. [Crossref] [PubMed]
- Blackhall FH, Shepherd FA. Angiogenesis inhibitors in the treatment of small cell and non-small cell lung cancer. Hematol Oncol Clin North Am 2004;18:1121-41. ix. [Crossref] [PubMed]
- Sharp A, Bhosle J, Abdelraouf F, et al. Development of molecularly targeted agents and immunotherapies in small cell lung cancer. Eur J Cancer 2016;60:26-39. [Crossref] [PubMed]
- Bunn PA Jr, Minna JD, Augustyn A, et al. Small Cell Lung Cancer: Can Recent Advances in Biology and Molecular Biology Be Translated into Improved Outcomes? J Thorac Oncol 2016;11:453-74. [Crossref] [PubMed]
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47-53. [Crossref] [PubMed]
- Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015;12:445-64. [Crossref] [PubMed]
- Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012;2:798-811. [Crossref] [PubMed]
- Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet 2010;376:235-44. [Crossref] [PubMed]
- Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 2010;376:245-51. [Crossref] [PubMed]
- Pleasance ED, Stephens PJ, O'Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184-90. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [Crossref] [PubMed]
- Ott PA, Fernandez ME, Hiret S, et al. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol 2015;33:abstr 7502.
- Duraiswamy J, Kaluza KM, Freeman GJ, et al. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013;73:3591-603. [Crossref] [PubMed]
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543-53. [Crossref] [PubMed]
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Gaponova AV, Nikonova AS, Deneka AY, et al. A Novel HSP90 Inhibitor-Drug Conjugate to SN38 Is Highly Effective in Small Cell Lung Cancer. Clin Cancer Res 2016;22:5120-9. [Crossref] [PubMed]
- Sevinc A, Kalender ME, Altinbas M, et al. Irinotecan as a second-line monotherapy for small cell lung cancer. Asian Pac J Cancer Prev 2011;12:1055-9. [PubMed]
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85-91. [Crossref] [PubMed]
- Lara PN Jr, Natale R, Crowley J, et al. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomic results from SWOG S0124. J Clin Oncol 2009;27:2530-5. [Crossref] [PubMed]
- Chen G, Huynh M, Fehrenbacher L, et al. Phase II trial of irinotecan and carboplatin for extensive or relapsed small-cell lung cancer. J Clin Oncol 2009;27:1401-4. [Crossref] [PubMed]
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43. [Crossref] [PubMed]
- Saunders LR, Bankovich AJ, Anderson WC, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7:302ra136. [Crossref] [PubMed]
- Rudin CM, Pietanza MC, Bauer TM, et al. Safety and efficacy of single-agent rovalpituzumab tesirine (SC16LD6.5), a delta-like protein 3 (DLL3)-targeted antibody-drug conjugate (ADC) in recurrent or refractory small cell lung cancer (SCLC). J Clin Oncol 2016;34:abstr LBA8505.
- Whiteman KR, Johnson HA, Mayo MF, et al. Lorvotuzumab mertansine, a CD56-targeting antibody-drug conjugate with potent antitumor activity against small cell lung cancer in human xenograft models. MAbs 2014;6:556-66. [Crossref] [PubMed]
- Belani CP, Dahlberg SE, Rudin CM, et al. Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E1508). Cancer 2016;122:2371-8. [Crossref] [PubMed]
- Rudin CM, Hann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 2012;18:3163-9. [Crossref] [PubMed]
- Pujol JL, Carestia L, Daurès JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8-15. [PubMed]


