Diagnostic performance of core needle biopsy in identifying breast phyllodes tumors
Introduction
Breast tumor is still a major health burden for Chinese women, especially in urban areas (1,2), among them, phyllodes tumors of the breast (PTB) is a clinically rare type of breast tumor, accounting for 2.0–3.0% of breast fibroepithelial tumors and only 0.3–1.0% of all breast tumors (3-5). PTB has a propensity to occur in females 35–55 years old (6), exhibits diverse biological behaviors, and has a high rate of local recurrence (5,7,8).
There are tremendous controversies regarding the nomenclature, classification (5-10), and biological traits of PTB. There are over 60 terms for this type of tumor in the literature, including phyllodes tumor, pseudo-sarcoma adenoma, mucus gland tumor, breast mixed tumor, and pseudo-sarcoma. It was depicted as a type of neoplasia with the appearance of fish meat, sections with cystic lobulation, rapid growth, and giant tumor sizes. The author also stressed that it was benign, a property that was questioned by Norris (9), who reported its potential malignancy and proposed to divide the tumor into benign and malignant types. In 2003, the International Histological Classification of Tumors of the World Health Organization (WHO) proposed the name phyllodes tumor and classified the tumors into benign, borderline, and malignant histologic types (3): (I) benign phyllodes tumors are characterized by expansive tumor growth, a clear tumor boundary, prominent proliferation and sparse arrangement of interstitial cells, an absence of or mild atypia, a mitotic count of 0–4 per 10 high power fields (HPFs), and a lack of bleeding or necrosis; (II) borderline phyllodes tumors are characterized by expansive growth or partially invasive growth of tumors, multi-angular growth of interstitial cells, a medium level of atypia, a mitotic count of 5–9 per 10 HPFs, and a minor level of bleeding and necrosis; and (III) malignant phyllodes tumors are characterized by invasive tumor growth into neighboring tissues, an unclear tumor boundary, excessive growth of interstitial cells, apparent atypia (possibly accompanied by heterologous differentiation), a mitotic count of ≥10 per 10 HPFs, and massive bleeding and visible necrosis. This nomenclature and associated classification criteria have been gradually accepted and employed globally.
PTB have the following properties: frequently lobulated sections, valgus deformities, tough textures, gray-white or gray-yellow color, commonly visible narrow clefts or cysts of various sizes, and clear or bloody fluids or jelly-like materials inside the tumors. In addition, the solid parts of these tumors often have a braided appearance or manifest as polylobed, tissue-oppressing cysts and also have focal hemorrhage, necrosis, and cystic degeneration. The microscopic exam of PTB has two components: the interstitial substance and the epithelium. The epithelial components are benign and often form ducts or overlay the surface of cysts and clefts. The real tumor components are hyperproliferative interstitial cells, also known as fibroblasts. These cells lose their normal arrangement and instead manifest braided, mesh-like, or spiral patterns. Tumor cells may exhibit a homogenous and diffuse distribution but may have variable levels of regional densities. Tumor cells are associated with differing degrees of atypia and mitotic counts. In addition, the tumors may show mucoid degeneration, or hemorrhagic necrosis. The epithelial components exhibit differing levels of abundance and are correlated with the interstitial components; the poorer the interstitial component is, the less the epithelial component is present. Recurrent tumors have a similar histology or are more malignant, and metastases only contain malignant interstitial components. Given the diversity of PTB in histologic variations and traits, it is challenging to clearly diagnosis and formulate appropriate treatment strategies for it.
Preoperative imaging and biopsy are the basis of preliminary judgment and classification for PTB. However, the breast diagnostic methods currently available do not distinctly reveal PTB and have a high misdiagnosis rate, which compromises the development of appropriate therapeutic plans and accurate assessments of prognosis (11-16). Among various preoperative auxiliary examinations, mammogram, ultrasound, and fine needle aspiration (FNA) all have a poor diagnostic performance for PTB (12-15). Core needle biopsy (CNB) is considered the most valuable auxiliary test for PTB that is currently available. It boasts accuracy, speed, simplicity-to-operate, minimal invasiveness, low rates of complications, aesthetically acceptable results, and an affordable cost. In comparison with other breast diagnostic methods, CNB is a relatively reliable auxiliary test for the preoperative diagnosis of PTB, although it still has a poorer diagnostic performance for this type of tumor. There are considerable variations in the accurate diagnosis rates of CNB for PTB, but most are below 50% (12-14). The main cause for the low rate of accurate diagnosis lies in the considerable similarities of PTB with other types of fibroepithelial lesions, in particular giant fibroadenoma, in clinical manifestations and imaging or histologic traits; these similarities inevitably result in extraordinary difficulty for its identification. Most cases of PTB, particularly benign and borderline PTB (with unapparent malignant manifestations), do not exhibit lobulation or cysts and contain no or very little epithelial components under preoperative CNB microscopy. Therefore, the results often suggest fibroadenoma or fibroepithelial lesions, and this uncertainty in the preoperative diagnosis makes it difficult to choose the correct surgical plan.
This retrospective study examined 128 PTB patients who received preoperative CNB and underwent breast surgery at Fudan University Shanghai Cancer Center from January 1, 2002 to April 1, 2013 and analyzed their clinical data. The results allowed us to compare the diagnostic performance of preoperative CNB and postoperative pathological analysis to evaluate the auxiliary usefulness of preoperative CNB for the diagnosis of PTB.
Methods
Patients
Between January 1, 2002 and April 1, 2013, a total of 454 PTB patients were treated at Fudan University Shanghai Cancer Center. Study patients were recruited using the following inclusion criteria. First, the primary and recurrent cases of PTB were treated at Fudan University Shanghai Cancer Center between January 1, 2002 and April 1, 2013, and the primary tumor resection specimens of recurrent cases were re-diagnosed by the pathologist. Second, the patient’s complete clinical history and pathological diagnostic data were available. Third, the surgical pathological diagnosis was based on the pathohistological classification standard for PTB, issued by WHO in 2003 (3). Fourth, the patient’s basic information included current phone numbers and addresses needed for follow-up visits. The exclusion criteria were as follows. First, the patient was treated at Fudan University Shanghai Cancer Center, but they did not have a primary tumor resection specimen examined. Second, the patient had other types of concurrent malignant tumors. Third, clinical data from the patient was lost. Fourth, follow-up visits were not possible due to a change of phone number or address or refusal due to other personal reasons. Based on these criteria, this study enlisted 128 patients who underwent preoperative CNB and postoperative pathological diagnosis at Fudan University Shanghai Cancer Center, whereby the accordance between the two diagnostic methods was analyzed. This retrospective study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center but did not require the patients to sign an informed consent form due to the retrospective nature of the study.
Diagnostic methods
CNB was performed by professionals at the Breast Diagnostic Center of Fudan University Shanghai Cancer Center and was guided by a GE logiq-7 color ultrasound machine with a probe frequency of 5–10 MHz. A Bard PG 522 MAGN-UM biopsy instrument was used for biopsy, with a 14 G needle (18 mm internal diameter, 20 mm outer diameter, and 20–22 mm needle groove). A suitable position and a puncture site were chosen based on the target. Once a complete lesion image was revealed by ultrasound, the insertion depth of the biopsy needle was determined based on lesion depth, size, and neighboring tissue. The biopsy specimens were placed in fixation solution before being sent for pathological examination. All 128 subjects received surgical treatments within 1 month after CNB examination for their breast tumors and were diagnosed with PTB based on postoperative pathological analyses. The histologic classification for pathological diagnosis was based on the following standards recommended by the WHO in 2003 (3).
The surgical biopsy sections were reviewed by two pathologists at Fudan University Shanghai Cancer Center. The following characteristics were reviewed: (I) histologic classification, specifically benign, borderline, or malignant; (II) tumor residue, specifically positive or negative; (III) mitotic count conducted in 10 consecutive HPFs in the area of greatest cell density, with results documented as 0–4/10 HPFs, 5–9/10 HPFs or ≥10/10 HPFs; (IV) hyperproliferation of interstitial cells with a documented area ratio of the tumor interstitial component per 1 HPF of 1/3 corresponding to mild, of 1/3–2/3 corresponding to intermediate, and of over 2/3 corresponding to severe; (V) atypia of interstitial cells, divided into mild, intermediate, or severe based on the size, morphology, and color of tumor interstitial cells; (VI) tumor boundary, specifically clear or invasive; (VII) tumor necrosis, specifically positive or negative; and (VIII) surgical margin, specifically negative or positive.
Follow-up
The follow-up were divided into three stages. First, the outpatient referral and follow-up records of the patients were retrieved using the electronic medical records system of Fudan University Shanghai Cancer Center. Next, a phone call was made to find out the postoperative visit and examination information of the patients. Lastly, for those patients whose phone numbers were invalid, follow-up letters were mailed to the given addresses. The main content of follow-up in the electronic medical records system included patient’s personal information, outpatient and inpatient records, preoperative examinations and reports, surgical records, detailed postoperative description, and diagnosis. The main content of phone or letter follow-up included important clinical history information unavailable in the electronic medical records system, detailed history of treatment events in other hospitals, frequency of postoperative review, the date and results of the last breast examination, recurrence or metastasis of tumor, and tumor-related death.
Statistical analysis
For the classification, statistical analysis, and comparison of clinicopathological data, t-tests were used for continuous variables and chi-square tests were used for classification data. An evaluation of the diagnostic tests was performed to compare the results of preoperative CNB and postoperative pathological examination. All tests were two-sided. P<0.05 was considered statistically significant. SPSS v22.0 software (IBM SPSS, NY, USA) was used for all statistical analyses and calculations.
Results
Baseline characteristics of included patients
All 128 PTB patients were females, with a mean age of 47 years (range, 12–67 years) and a median follow-up duration of 35 months (range, 11–96 months). Postoperative pathological examination after CNB revealed that among these cases, 41 had benign PTB (32.0%), 71 had borderline PTB (55.5%), and 16 had malignant PTB (12.5%). The patients’ clinicopathological traits are classified and summarized according to histologic types in Table 1. The differences between the tumor sizes of benign, borderline, and malignant types were statistically significant (P=0.001); the malignant type had the greatest diameter, whereas the benign type had the smallest. The surgical approach accepted by most patients was simple excision (SE), followed by wide local excision (WLE). Few patients underwent mastectomy (M, non-breast-conserving surgery), including simple mastectomy (SM) and modified radical mastectomy (MRM). With the exceptions of surgical margin and tumor residue, other pathological traits exhibited significant differences in distribution between the three histologic types (P<0.05).
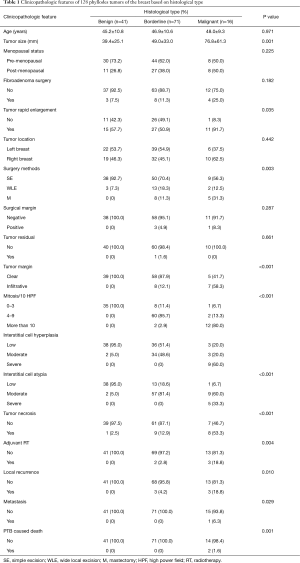
Full table
The clinicopathological traits of the 128 patients based on the CNB diagnosis of whether a subject had PTB are summarized in Table 2. CNB-reported PTB cases had greater tumor diameter than their CNB-reported non-PTB counterparts (64.7 vs. 47.1 mm; P=0.047); in addition, significant differences were also present in the surgical history for fibroadenoma, surgical approaches, and histological staging. Of the pathological traits, significant differences were present in the mitotic counts, hyperproliferation of interstitial cells, atypia degree of interstitial cells, and tumor necrosis between the PTB-diagnosed and non-PTB subjects (P<0.05), whereas no significant differences were found in the surgical margins, tumor residues, and tumor boundaries.
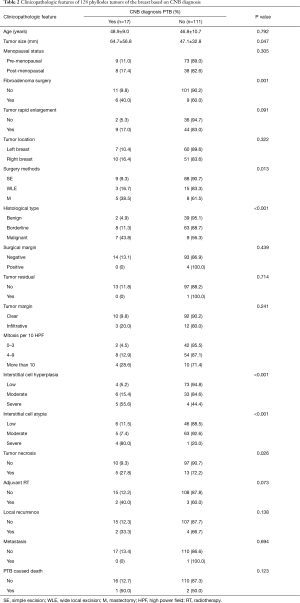
Full table
Comparison of the diagnostic results between CNB and postoperative pathology
The CNB diagnostic results of 128 patients are summarized in Table 3. The results revealed that 17 patients were diagnosed with phyllodes tumor via CNB, yielding a diagnosis accuracy rate of 13.3%. Of these, 9 were diagnosed with benign PTB (7.0%), 4 with borderline PTB (3.1%), and 4 with malignant PTB (3.1%). In addition, CNB diagnosis reported 25 cases of fibroadenoma (FA, 19.5%), 73 cases of fibroepithelial lesions (FEL, 57.0%), 10 cases of breast disease (7.8%), and 3 undetermined cases (2.4%). For the three undetermined cases, CNB diagnosis did not provide pathological properties due to inadequate biopsy materials; whether tumors were present required further examination.
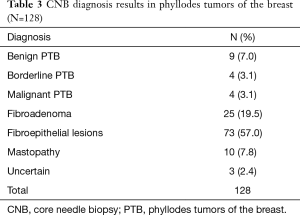
Full table
Comparisons between CNB diagnosis and postoperative pathological examination are summarized in Table 4. Based on preoperative CNB, PTB cases were often initially suggested to be FA or FEL, regardless of their benign, borderline, or malignant status as revealed via postoperative pathology. The remaining options of CNB diagnosis included other breast disease or undetermined, as shown in Table 4. Of the 41 cases of benign PTB, 14 (34.1%) were initially diagnosed with FA and 21 (51.2%) were initially diagnosed with FEL via CNB. Of the 71 cases of borderline PTB, 11 (15.5%) were initially diagnosed with FA and 45 (63.4%) were initially diagnosed with FEL via CNB. Of the 16 cases of malignant PTB, 7 (43.7%) were initially diagnosed with FEL via CNB.

Full table
Of the 128 cases, 17 were initially diagnosed with PTB via CNB (Table 5). These included 9 cases in which the CNB results completely matched those of the postoperative pathology, yielding an accuracy rate of 7.0% (9/128). Of the 9 cases of benign PTB diagnosis via CNB, 2 (22.2%) were verified by postoperative pathology, whereas 5 (55.6%) and 2 (22.2%) cases were revealed to be borderline PTB and malignant PTB, respectively. Of the 4 cases diagnosed with borderline PTB via CNB, 3 (75.0%) were verified by postoperative pathology, whereas 1 (25.0%) was revealed to be malignant PTB. All 4 cases of malignant PTB diagnosis via CNB were verified by postoperative pathology (100%). Therefore, CNB diagnosis did not determine 9.8% (4/21) of benign PTB cases, 9.9% (7/71) of borderline PTB cases, and 12.5% (2/16) of malignant PTB cases.

Full table
Tables 6-8 summarize the diagnostic results of CNB for the three types of PTB. The “other” category in the three tables corresponds to the non-benign, non-borderline, and non-malignant PTBs that were indicated by CNB or postoperative pathology. Evaluations of the diagnostic performance of CNB on different histologic types of PTB are summarized in Table 9. The sensitivities of CNB in diagnosing benign, borderline, and malignant PTB were 4.9%, 4.2%, and 25.0%, respectively, whereas the corresponding specificities were 92.0%, 98.2%, and 100%, respectively. In addition, the positive predictive values (PPVs) for benign, borderline, and malignant PTB were 22.2%, 75.0%, and 100%, respectively, whereas the corresponding negative predictive values (NPVs) were 67.2%, 45.2%, and 90.3%, respectively. The accuracy rates were 64.1%, 46.1%, and 90.6% for benign, borderline, and malignant PTB, respectively, and the corresponding Youden Index (YIs) were −3.1%, 2.4%, and 25.0%, respectively.

Full table

Full table

Full table

Full table
Discussion
The wide application of preoperative CNB is intended to provide a reliable pathological basis for formulating appropriate tumor treatment plans. However, the available literature reveals that although CNB showed greater accuracy than other methods such as ultrasound, mammogram, and MRI in diagnosing PTB (17,18), it still has limited clinical significance (12,19-21). Consistent with previous studies, the diagnostic accuracy of CNB for the 128 subjects in this study was only 13.3% (17/128). Based on the CNB diagnostic results, 76.5% of the cases were FA (19.5%) and FEL (57.0%) (Table 3). Among the 128 PTB patients, only 4.9% (2/41) of the benign cases, 4.2% (3/71) of the borderline cases, and 25.0% (4/16) of the malignant cases had consistent results between CNB and postoperative pathology (Table 4). Lastly, 22.2% (2/9) of the benign cases, 75.0% (3/4) of the borderline cases, and 100% (4/4) of the 17 malignant cases reported by CNB were later verified by postoperative pathology (Table 5).
Since 2003, studies on the accuracy of auxiliary CNB diagnosis have reported different results (Table S1). Bode et al. (11) reported a CNB accuracy rate of 83.0%, the highest among all relevant studies. Choi (12) reported that among 129 patients having undergone preoperative CNB diagnosis, 104 were verified by postoperative pathology, yielding an accuracy rate of 80.6%, similar to the results of Bode et al. (11). In addition, Komenaka et al. (22) and Ward et al. (17) reported accurate CNB diagnostic rates of 76.0% and 63.0%. Although these four reports achieved diagnosis rates above 50%, most studies reported accuracies below this line (15,19,20,23-25). The lowest reported accurate CNB diagnosis rate of PTB was only 13.3%. The differences in diagnosis rates may be attributed to differences in CNB apparatuses or operational skills, but these differences may also stem from the objectives and methods of individual studies, including sample size, cohort characteristics, the presence or absence of an FA control group, and the presence or absence of a review of the CNB results. Bolívar et al. (26) proposed obtaining a minimum of three specimens via ultrasound-guided CNB for cases of breast tumors that were difficult to examine via palpation. Bode et al. (11) reported that using 14 G or 16 G biopsy needles could deliver a relatively improved outcome in differentiating a PTB specimen from that of FA. Although 14 G needles were used in this study, only 1 specimen was obtained from each breast tumor patients, which might explain the relatively low rate of accurate CNB diagnosis. In summary, the following three crucial factors may account for the lower CNB diagnosis rates found in this study compared with those of previous studies. First, all 128 subjects in this study were confirmed to be PTB patients based on postoperative pathology, with no FA patients recruited as a control. Second, the patients in this study were all treated at Fudan University Shanghai Cancer Center, which might diminish representation; this limitation could be resolved by using a multi-center study with a greater sample size. Third, although the postoperative pathological specimens of the 128 subjects were reviewed by pathologists using several indicators, including pathological diagnosis, mitotic count, and atypia of interstitial cells, the preoperative CNB specimens were not reviewed.
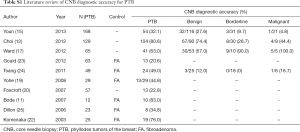
Full table
There was a significant difference (P=0.047) in the tumor diameter between the 17 CNB-reported PTB cases (64.7 mm) and the 121 CNB-reported non-PTB counterparts (47.1 mm). Of the 15 cases with prior fibroadenoma surgical history, 6 (40.0%) were reported to have PTB via CNB, whereas of the 112 subjects without FA surgical history, 11 (9.8%) were reported to have PTB via CNB; this difference was significant (P=0.001). Of the 53 patients whose main symptom was rapid tumor enlargement, 9 (17.0%) were reported to have PTB via CNB, whereas of the 38 patients whose main symptom was not rapid tumor enlargement, 2 (5.3%) were reported to have PTB via CNB; this difference was significant (P=0.091). These results suggest that patients with large tumor sizes, rapid tumor enlargement, or FA surgical history are more likely to obtain correct diagnosis via preoperative CNB. Ward et al. (17) proposed that age was a factor in CNB examination, as they found that young patients were more likely to receive false negatives. In addition, the authors also argued that a relatively large tumor size and a history of rapid tumor enlargement were two factors that supported PTB diagnosis in CNB examination and were worth considering. Therefore, combining CNB pathology with clinical history can improve the diagnostic accuracy of CNB, particularly when it is difficult to determine whether a tumor tissue is PTB or another type based on the microscopy of biopsy specimens. It is also important that a suggestion is made to remind clinicians to perform other tests along with the CNB diagnostic results to verify PTB.
The primary test for patients who are found to have breast tumors via physical examination are imaging examinations, such as ultrasound, which, if necessary, are followed by CNB. Currently, imaging combined with CNB is the main approach for diagnosing breast tumors. In another retrospective study reported by our group, 367 out of 404 PTB patients had complete preoperative imaging data (Table S2). Among them, 132 subjects received only breast ultrasound, which generated a PTB diagnosis rate of 10.6% (14/132), and 38 subjects received only mammogram, which delivered a PTB diagnosis rate of 0%. Furthermore, of 145 subjects who received both ultrasound and mammogram, 21 (14.5%) obtained positive PTB results via at least one examination; of 15 subjects who received ultrasound and MRI, 6 (40.0%) were diagnosed with PTB; of 36 subjects who received all 3 imaging examinations, 11 (30.6%) had indications of PTB. These results suggest that whereas using a single imaging test generates a very low diagnostic rate for PTB, the integration of two or more tests can significantly enhance the diagnosis rate. Among the 128 subjects examined in this study, 6 did not have complete imaging data. For the remaining 122 subjects, 18 (14.8%) had indications of PTB from at least one test, but only 6 received CNB identification of PTB. Of the 104 patients who received no indications of PTB from any imaging tests, only 9 (8.7%) were diagnosed with CNB (Table S3). Overall, only 27 (22.1%) of the 122 patients received positive results of PTB from CNB or at least 1 imaging test. Therefore, these results suggest that although the consistency in PTB diagnosis between imaging tests and CNB examination was very low, their integration could boost diagnostic accuracy; this finding is in agreement with previous studies by Ward et al. (17), Gould et al. (23), and Gatta et al. (18).

Full table

Full table
Further analysis of the 128 patients revealed that 85.3% (35/41) of the benign PTB cases, 78.9% (56/71) of the borderline PTB cases, and 43.7% (7/16) of the malignant PTB cases were diagnosed with FA or FEL. Our evaluation of these outcomes (Table 9) revealed that CNB performed better at diagnosing malignant PTB cases compared with borderline and benign cases in a variety of aspects, including accuracy (90.6%), sensitivity (25.0%), specificity (100%), positive predictive value (100%), and negative predictive value (90.3%). In addition, the Youden Index of CNB diagnosis was 2.4% and 25.0% for borderline and malignant PTB, respectively, but was only −3.1% for benign PTB. These results suggest that CNB has no clinical significance at diagnosing benign PTB. Clinically, it is crucial to distinguish FA from PTB; however, this is a daunting task for both imaging tests and CNB. Differentiation is important due to the different surgical approaches advised for these tumors. FA can be treated with minimally invasive surgeries (27), as the residual tumor tissue has little risk of recurrence and may even gradually shrink with time. By contrast, if a PTB tumor is not completely excised with a negative surgical margin, postoperative recurrence may quickly ensue; for malignant PTB cases, metastasis may occur (28). It has been reported that CNB in conjunction with pathological differentiation between FA and PTB may increase the diagnostic accuracy of CNB for PTB (14,24,29,30). The differential traits of FA and PTB include mitotic count, atypia of interstitial cells, hyperproliferation of interstitial cells, and clear or invasive tumor boundary. Tsang et al. (24) reported a comparative study on the CNB histology of 49 cases of PTB and 69 cases of FA, all of which received CNB examination and were verified via postoperative pathology. The results revealed that among various CNB pathological results, mitotic count, hyperproliferation of interstitial cells, and atypia of interstitial cells were the key factors that could distinguish FA from PTB, as these indicators exhibited a high consistency between CNB results and postoperative pathological results (Pearson correlation coefficient >0.6, P<0.01). Lee et al. (14) argued that interstitial components are the key indicator of malignant PTB in CNB-based diagnosis, with hyperproliferation and atypia of interstitial cells being the most important factors for identifying PTB. Likewise, Morgan et al. (29), Jara-Lazaro et al. (30), and Yohe et al. (19) all reported that mitotic count was a vital factor for the auxiliary discrimination of PTB, whereas Jara-Lazaro et al. (30) also found that a blurred tumor boundary upon CNB examination was a reliable factor for the diagnosis of PTB.
In summary, CNB-derived data provides a pathological basis to assist the preoperative diagnosis of PTB. Because phyllodes tumors are characterized by elaborate histologic features that are microscopically similar to other types of FEL and FA, CNB has a poor diagnostic performance and correspondingly limited clinical significance for PTB. An integrated approach involving clinical manifestations, imaging data, and CNB pathology may be a more reliable strategy for the preoperative diagnosis of PTB. Importantly, our data suggest that patients with positive CNB-based PTB results along with a surgical history of fibroadenoma and rapid tumor enlargement have a significantly increased likelihood of verifiable PTB.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center but did not require the patients to sign an informed consent form due to the retrospective nature of the study.
References
- Jia M, Zheng R, Zhang S, et al. Female breast cancer incidence and mortality in 2011, China. J Thorac Dis 2015;7:1221-6. [PubMed]
- Levaggi A, Poggio F, Lambertini M. The burden of breast cancer from China to Italy. J Thorac Dis 2014;6:591-4. [PubMed]
- Tavassoéli FA, Devilee P. Pathology and Genetics Tumours of the Breast and Female Genital Organs. World Health Organization, international agency for research on cancer, international academy of pathology 2003.
- Reinfuss M, Mituś J, Duda K, et al. The treatment and prognosis of patients with phyllodes tumor of the breast: an analysis of 170 cases. Cancer 1996;77:910-6. [Crossref] [PubMed]
- Guerrero MA, Ballard BR, Grau AM. Malignant phyllodes tumor of the breast: review of the literature and case report of stromal overgrowth. Surg Oncol 2003;12:27-37. [Crossref] [PubMed]
- Guillot E, Couturaud B, Reyal F, et al. Management of phyllodes breast tumors. Breast J 2011;17:129-37. [Crossref] [PubMed]
- Geisler DP, Boyle MJ, Malnar KF, et al. Phyllodes tumors of the breast: a review of 32 cases. Am Surg 2000;66:360-6. [PubMed]
- Böcker W. WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics. Verh Dtsch Ges Pathol 2002;86:116-9. [PubMed]
- Norris HJ, Taylor HB. Relationship of histologic features to behavior of cystosarcoma phyllodes. Analysis of ninety-four cases. Cancer 1967;20:2090-9. [Crossref] [PubMed]
- Karim RZ, Gerega SK, Yang YH, et al. Phyllodes tumours of the breast: a clinicopathological analysis of 65 cases from a single institution. Breast 2009;18:165-70. [Crossref] [PubMed]
- Bode MK, Rissanen T, Apaja-Sarkkinen M. Ultrasonography and core needle biopsy in the differential diagnosis of fibroadenoma and tumor phyllodes. Acta Radiol 2007;48:708-13. [Crossref] [PubMed]
- Choi J, Koo JS. Comparative study of histological features between core needle biopsy and surgical excision in phyllodes tumor. Pathol Int 2012;62:120-6. [Crossref] [PubMed]
- El Hag IA, Aodah A, Kollur SM, et al. Cytological clues in the distinction between phyllodes tumor and fibroadenoma. Cancer Cytopathol 2010;118:33-40. [Crossref] [PubMed]
- Lee AH, Hodi Z, Ellis IO, et al. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology 2007;51:336-44. [Crossref] [PubMed]
- Youn I, Choi SH, Moon HJ, et al. Phyllodes tumors of the breast: ultrasonographic findings and diagnostic performance of ultrasound-guided core needle biopsy. Ultrasound Med Biol 2013;39:987-92. [Crossref] [PubMed]
- Tallet A, Rua S, Jalaguier A, et al. Impact of preoperative magnetic resonance imaging in breast cancer patients candidates for an intraoperative partial breast irradiation. Transl Cancer Res 2015;4:148-54.
- Ward ST, Jewkes AJ, Jones BG, et al. The sensitivity of needle core biopsy in combination with other investigations for the diagnosis of phyllodes tumours of the breast. Int J Surg 2012;10:527-31. [Crossref] [PubMed]
- Gatta G, Iaselli F, Parlato V, et al. Differential diagnosis between fibroadenoma, giant fibroadenoma and phyllodes tumour: sonographic features and core needle biopsy. Radiol Med 2011;116:905-18. [Crossref] [PubMed]
- Yohe S, Yeh IT. "Missed" diagnoses of phyllodes tumor on breast biopsy: pathologic clues to its recognition. Int J Surg Pathol 2008;16:137-42. [Crossref] [PubMed]
- Foxcroft LM, Evans EB, Porter AJ. Difficulties in the pre-operative diagnosis of phyllodes tumours of the breast: a study of 84 cases. Breast 2007;16:27-37. [Crossref] [PubMed]
- Wang Y. Development of cancer diagnostics—from biomarkers to clinical tests. Transl Cancer Res 2015;4:270-9.
- Komenaka IK, El-Tamer M, Pile-Spellman E, et al. Core needle biopsy as a diagnostic tool to differentiate phyllodes tumor from fibroadenoma. Arch Surg 2003;138:987-90. [Crossref] [PubMed]
- Gould DJ, Salmans JA, Lassinger BK, et al. Factors associated with phyllodes tumor of the breast after core needle biopsy identifies fibroepithelial neoplasm. J Surg Res 2012;178:299-303. [Crossref] [PubMed]
- Tsang AK, Chan SK, Lam CC, et al. Phyllodes tumours of the breast - differentiating features in core needle biopsy. Histopathology 2011;59:600-8. [Crossref] [PubMed]
- Dillon MF, Quinn CM, McDermott EW, et al. Needle core biopsy in the diagnosis of phyllodes neoplasm. Surgery 2006;140:779-84. [Crossref] [PubMed]
- Bolívar AV, Alonso-Bartolomé P, García EO, et al. Ultrasound-guided core needle biopsy of non-palpable breast lesions: a prospective analysis in 204 cases. Acta Radiol 2005;46:690-5. [Crossref] [PubMed]
- Cant PJ, Madden MV, Close PM, et al. Case for conservative management of selected fibro-adenomas of the breast. Br J Surg 1987;74:857-9. [Crossref] [PubMed]
- Parker SJ, Harries SA. Phyllodes tumours. Postgrad Med J 2001;77:428-35. [Crossref] [PubMed]
- Morgan JM, Douglas-Jones AG, Gupta SK. Analysis of histological features in needle core biopsy of breast useful in preoperative distinction between fibroadenoma and phyllodes tumour. Histopathology 2010;56:489-500. [Crossref] [PubMed]
- Jara-Lazaro AR, Akhilesh M, Thike AA, et al. Predictors of phyllodes tumours on core biopsy specimens of fibroepithelial neoplasms. Histopathology 2010;57:220-32. [Crossref] [PubMed]


