Orchestration of the crosstalk between astrocytes and cancer cells affects the treatment and prognosis of lung cancer sufferers with brain metastasis
Introduction
Lung cancer is one of the most common malignant tumors and remains the leading cause of cancer-related lethality worldwide (1,2). As the common site of extra-thoracic distant metastasis of lung cancer, brain is most reported for the poor prognosis (3). Multistage steps are implicated in the establishment of clinically relevant brain metastasis (4). The classical seed and soil hypothesis enlightens that the successful growth of metastatic cells are dependent on the interactions and characteristics of cancer cells (seeds) and their target organs (soil) (5). As it explicates, circulating cancer cells must overcome many obstacles to colonize in brain (6). However, once the distinctive biology of metastases is established in brain, the host microenvironment will play a “sanctuary” role in promoting metastatic growth and treatment resistance (7), and then current treatments frequently fail to provide durable responses (8). An improved understanding of the crosstalk between microenvironment and cancer cells is needed to prevent and treat metastatic cancer (9).
Astrocytes participate in the repair and scarring of the brain following injuries and are pretty crucial for microenvironment homeostasis. Dysregulation of astrocytes is thought to contribute to the pathogenesis of brain metastasis progression (10). In brain metastases, an abundance of activated astrocytes were found within the lesion (11). Cancer cells that infiltrate the brain are immediately exposed to astrocytes in the perivascular space and produce deleterious signals to repel invading cells (6). Cancer cells must be shielded from such signals in order to survive and to extract benefits from the stroma, including benefits from astrocytes (12).
Massagué and his colleagues two opposite roles for astrocytes in brain metastasis in separate works: dispelling cancer cells entering the brain parenchyma (7,13) and promoting the progression of metastasis in the brain (8,14). The contact with astrocytes leads to up-regulated expression of multiple genes in the cancer cells (15), including several survival genes that are in charge of the increased resistance of cancer cells to cytotoxic drugs (12). Better therapeutic strategies need to be raised urgently.
Very recently, Lee et al. proposed that astrocytes protected cancer cells from paclitaxel. They demonstrated that the protection effect could be eliminated by an endothelin (ET) receptor antagonist that modulates the crosstalk between astrocytes and cancer cells (11). These findings have shed light on the combination of crosstalk modulators with anti-cancer drugs in brain metastasis treatment.
Crosstalk that suppress the progression of metastasis
Specialized mechanisms are required for cancer cells to penetrate the blood-brain barrier (BBB), and most cancer cells that pass through the BBB will die (16). What kills most cancer cells that pass the BBB remains a question of biologic and clinical interest. Astrocytes have an integral role in preserving BBB properties, they support endothelial cells and imped cancer cells from traversing into the brain (17). Researchers noticed that only cells that grew on top of blood capillaries were able to survive, and each cell stick very closely to its vessel, just like a panda bear hugging a tree trunk. This hugging is indispensable, if a cancer cell detaches from its vessel, it will be killed by astrocytes nearby (7,16).
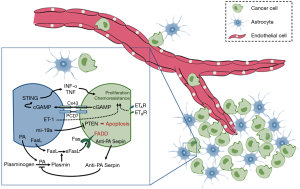
Astrocytes kill the infiltrated cancer cells by secreting a pro-apoptotic cytokine protein called Fas ligand (FasL) (18) that is highly expressed in reactive astrocytes in ischemia, Alzheimer’s disease, encephalomyelitis, brain trauma, and multiple sclerosis (19). As early as 2008, it was demonstrated that plasmin cleaved FasL at Arg144, releasing sFasL as a diffusible cell death signal (20). Brain metastatic cells are susceptible to apoptosis if they are exposed to sFasL expressed by reactive astrocytes in the brain parenchyma (21).
Apart from converting FasL into a death signal for cancer cells, plasmin suppresses brain metastasis in another way. They inactivate L1CAM (22,23), a molecule expressed by metastatic cells for metastatic outgrowth and for spreading along brain capillaries. Both tissue plasminogen activator PA (tPA) and urokinase plasminogen activator (uPA) are implicated in the production of plasmin, they convert plasminogen into plasmin (24,25). Early studies found that lung cancer brain metastasis were infiltrated by reactive astrocytes, which are major sources of plasminogen activators (PAs) in ischemia and neurodegenerative injury (26). Abundant reactive astrocytes in the metastases lead to a resultant production of plasmin, which induce parent lung cancer cells to undergo apoptosis.
Crosstalk that facilitate the progression of metastasis
Brain metastatic cells suffer Fas-dependent death (FADD) in the brain unless they are protected by anti-PA serpins (21). To prevent the generation of plasmin and its suppressive effects in metastasis, high levels of anti-PA serpins, are expressed by the survived cancer cells, including neuroserpin (NS) and serpin B2. The up-regulation of NS and serpin B2 in brain metastatic cells was confirmed at the protein level. Gene- expression data showed that the expression of SERPINI1 and SERPINB2, which encode NS and serpin B2 respectively, in the tumors was associated with brain relapse. In a word, anti-PA serpins provide a unified mechanism for the initiation of brain metastasis in lung cancer.
It seems that cancer cells that survive from the pro-survival signals such as anti-PA serpins will proliferate without a hitch (6), just like seeds find their appropriate soil. Chen et al. showed that it result from the establishment of gap junctions between cancer cells and reactive astrocytes (27). Researchers observed that cancer cells and astrocytes communicate with each other through gap junctions, and the communication activates pro-survival signals consistently. What’s more, ions pass through gap junctions, and it was demonstrated as a potential mechanism of chemoresistance in lung cancer cells (9). It is shown that by means of protocadherin 7 (PCDH7), metastatic cells selectively form connexin 43 (Cx43) gap junctions with astrocytes, then cyclic GMP-AMP (cGAMP) is allowed to pass from cancer cells to astrocytes to activate STING, an inherent immune response pathway to cytosolic double-stranded DNA (dsDNA). The resulting production of tumour necrosis factor (TNF) and interferon-α (IFNα) in astrocytes supports outgrowth and drug-resistance in metastatic cells (27).
In addition to the direct interactions as gap junctions, astrocytes can reprogram cancer cells through epigenetically regulators. It is shown that co-cultured cancer cells with murine astrocytes significantly reduced the expression of PTEN in cancer cells, and both human and murine tumor cells with normal PTEN expression lose the expression of PTEN after infiltration into the brain, but not to other organs. This, reversible, brain microenvironment dependent down-regulation of PTEN is regulated by microRNAs from astrocytes in the brain (28). This evidence provided a mechanism in which astrocytes induce a reversible loss of PTEN, an important tumor suppressor (29), in metastatic cancer cells.
Crosstalks that enhance drug resistance of metastases
Drug resistance invariably limits the clinical efficacy of chemotherapy against brain metastasis. It has been generally acknowledged that drug resistance is mainly attributed to the impermeable structure of the BBB (30) and the expression of P-glycoprotein by cancer cells (31), but recently a novel mechanism illustrated that crosstalk between activated cancer cells and astrocytes led to the increased drug resistance (3,32).
Researchers found that co-culture of astrocytes with lung cancer cells led to up-regulation of survival genes in the cancer cells (24), and the up-regulation degree was directly correlated with increased resistance to all tested chemotherapeutic agents. The direct cell-to-cell interaction between astrocytes and cancer cells altered the pattern of gene expression in both cancer cells and astrocytes, including BCL2L1, GSTA5, and TWIST1. We further discovered that the up-regulation of the survival genes and consequent resistance are transient owing to the direct contact between the astrocytes and cancer cells through gap junctions (12).
However, the mechanisms how astrocyte stimulates the expression of survival genes in cancer cells remains unknown. Recent studies have proposed that activated ET signaling may protect cancer cells from the chemotherapeutic drugs induced cytotoxicity (33). As mentioned previously, reactive astrocytes are the main sources of metastases suppressors such as FasL and PAs. To date, accumulating evidence suggests that up-regulation of ET is also a distinctive feature of the reactive astrocytes that accompanies the pathologies of CNS diseases (34), including brain metastasis. As early as 1995, it was found that peritumoral astrocytes overexpress ET in most of the human brain metastasis cases (35). Kim et al. found that co-incubation of astrocytes with cancer cells with led to up-regulated ET-1 expression in astrocytes and promoted the expression of ET receptors (ETAR and ETBR) on the cancer cells significantly. They demonstrated that astrocytes protect cultured cancer cells from paclitaxel through an ET-dependent signaling that leads to up-regulated expression of anti-apoptotic proteins in cancer cells. What’s more, the chemo-protective effect requires physical interaction between astrocytes and cancer cells can be abolished by antagonism of ETAR and ETBR signaling (33).
New evidence
Very recently, Lee et al. reported that macitentan, a dual ET receptor antagonist, abolished astrocyte-induced protection of cancer cells to paclitaxel and increased overall survival of mice with experimental brain metastases (11). They found that both clinical and experimental lung cancer brain metastasis were infiltrated and surrounded by glial fibrillary acidic protein positive astrocytes, and ETAR and ETBR were heterogeneously expressed in the clinical brain metastasis samples. Dual antagonism of ETAR and ETBR signaling with macitentan in combination with paclitaxel significantly increases overall survival of mice with experimental brain metastases compared to the treatment of paclitaxel alone. Such findings reveals that although astrocytes up-regulate anti-apoptotic proteins in cancer cells through an ET-dependent signaling mechanism which provides protection of cancer cells, the chemo-protective effects can be eliminated by macitentan through blocking ETAR and ETBR signaling. The combination therapy of crosstalk modulators and anti-cancer drugs may provide a new strategy for the treatment of patients with brain metastases.
Summary
Crosstalk between astrocytes and cancer cells demonstrate that astrocytes in the microenvironment affect the biologic behavior of tumor cells and strengthen the contention (Figure 1). To improve the therapeutic outcome, orchestration of the crosstalk in microenvironment is required in consideration of the complexity interaction between astrocytes and cancer cells. Pharmacological inhibition of gap junctions between cancer cells and astrocytes in mice was reported to suppress brain metastasis. There is no doubt that we must take tumor microenvironment into consideration when designing the therapy schedules. New evidence reported by Lee et al. applied the novel strategies into practice, drugs targeting the brain microenvironment and extrinsic mechanisms of resistance in cancer cells lead to beneficial effects in treating lung cancer brain metastasis. Therapies using cross-talk modulators may benefit the treatment of lung cancer brain metastasis in the future.
Acknowledgements
Funding: This work was supported by the Jiangsu Province Scientific Research Innovation Project of University graduate students (KYLX15_0979), the Innovation Team [No. LJ201123 (EH11)], grants from Key Academic Discipline of Jiangsu Province “Medical Aspects of Specific Environments”, and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231802).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lenka G, Tsai MH, Hsiao JH, et al. Overexpression of methylation-driven DCC suppresses proliferation of lung cancer cells. Transl Cancer Res 2016;5:169-75. [Crossref]
- Zheng R, Rao Y, Jiang H, et al. Therapeutic potential of Ginsenoside Rg3 via inhibiting Notch/HES1 pathway in lung cancer cells. Transl Cancer Res 2016;5:464-9. [Crossref]
- Fidler IJ. The Biology of Brain Metastasis: Challenges for Therapy. Cancer J 2015;21:284-93. [Crossref] [PubMed]
- Alderton GK. Metastasis: Directions to metastatic sites. Nat Rev Cancer 2015;15:696-7. [Crossref] [PubMed]
- Ramakrishna R, Rostomily R. Seed, soil, and beyond: The basic biology of brain metastasis. Surg Neurol Int 2013;4:S256-64. [PubMed]
- Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature 2016;529:298-306. [Crossref] [PubMed]
- Obenauf AC, Massagué J. Surviving at a distance: organ specific metastasis. Trends Cancer 2015;1:76-91. [Crossref] [PubMed]
- Obenauf AC, Zou Y, Ji AL, et al. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature 2015;520:368-72. [Crossref] [PubMed]
- Ferraro GB, Kodack DP, Askoxylakis V, et al. Closing the gap: astrocytes and brain metastasis. Cell Res 2016;2:973-4. [Crossref] [PubMed]
- Hoshide R, Jandial R. Conduits Between Cancer Cells and Astrocytes. Neurosurgery 2016;79:N16-7.
- Lee HJ, Hanibuchi M, Kim SJ, et al. Treatment of experimental human breast cancer and lung cancer brain metastases in mice by macitentan, a dual antagonist of endothelin receptors, combined with paclitaxel. Neuro Oncol 2016;18:486-96. [Crossref] [PubMed]
- Kim SJ, Kim JS, Park ES, et al. Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia 2011;13:286-98. [Crossref] [PubMed]
- Malladi S, Macalinao DG, Jin X, et al. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell 2016;165:45-60. [Crossref] [PubMed]
- David CJ, Huang YH, Chen M, et al. TGF-β Tumor Suppression through a Lethal EMT. Cell 2016;164:1015-30. [Crossref] [PubMed]
- Hanibuchi M, Kim SJ, Fidler IJ, et al. The molecular biology of lung cancer brain metastasis: an overview of current comprehensions and future perspectives. J Med Invest 2014;61:241-53. [Crossref] [PubMed]
- Vanharanta S, Massagué J. Origins of metastatic traits. Cancer Cell 2013;24:410-21. [Crossref] [PubMed]
- Wang L, Cossette SM, Rarick KR, et al. Correction: Astrocytes Directly Influence Tumor Cell Invasion and Metastasis In Vivo. PLoS One 2015;10:e0137369. [Crossref] [PubMed]
- Wang X, Haroon F, Karray S, et al. Astrocytic Fas ligand expression is required to induce T-cell apoptosis and recovery from experimental autoimmune encephalomyelitis. Eur J Immunol 2013;43:115-24. [Crossref] [PubMed]
- Choi K, Ni L, Jonakait GM. Fas ligation and tumor necrosis factor α activation of murine astrocytes promote heat shock factor-1 activation and heat shock protein expression leading to chemokine induction and cell survival. J Neurochem 2011;116:438-48. [Crossref] [PubMed]
- Bajou K, Peng H, Laug WE, et al. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell 2008;14:324-34. [Crossref] [PubMed]
- Valiente M, Obenauf AC, Jin X, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 2014;156:1002-16. [Crossref] [PubMed]
- Schröder C, Schumacher U, Fogel M, et al. Expression and prognostic value of L1-CAM in breast cancer. Oncol Rep 2009;22:1109-17. [PubMed]
- Schäfer MK, Altevogt P. L1CAM malfunction in the nervous system and human carcinomas. Cell Mol Life Sci 2010;67:2425-37. [Crossref] [PubMed]
- Ganesh BS, Chintala SK. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS One 2011;6:e18305. [Crossref] [PubMed]
- Adhami F, Yu D, Yin W, et al. Deleterious effects of plasminogen activators in neonatal cerebral hypoxia-ischemia. Am J Pathol 2008;172:1704-16. [Crossref] [PubMed]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 2010;119:7-35. [Crossref] [PubMed]
- Chen Q, Boire A, Jin X, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016;533:493-8. [Crossref] [PubMed]
- Zhang L, Zhang S, Yao J, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 2015;527:100-4. [Crossref] [PubMed]
- Chang XJ, Zuo XS, Wang ZT, et al. The clinical significance of loss of FHIT and PTEN expression in 289 patients with non-small-cell lung cancer. Transl Cancer Res 2016;5:294-301. [Crossref]
- Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J 2015;282:4067-79. [Crossref] [PubMed]
- Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 2010;16:5664-78. [Crossref] [PubMed]
- Bui L, Hendricks A, Wright J, et al. Brain Tumor Genetic Modification Yields Increased Resistance to Paclitaxel in Physical Confinement. Sci Rep 2016;6:26134. [Crossref] [PubMed]
- Kim SW, Choi HJ, Lee HJ, et al. Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro Oncol 2014;16:1585-98. [Crossref] [PubMed]
- Zhang M, Olsson Y. Hematogenous metastases of the human brain--characteristics of peritumoral brain changes: a review. J Neurooncol 1997;35:81-9. [Crossref] [PubMed]
- Zhang M, Olsson Y. Reactions of astrocytes and microglial cells around hematogenous metastases of the human brain. Expression of endothelin-like immunoreactivity in reactive astrocytes and activation of microglial cells. J Neurol Sci 1995;134:26-32. [Crossref] [PubMed]


