Bronchoalveolar neutrophilia inversely correlates with DLCO at diagnosis in asbestosis but not lung function decline at 1 year
Introduction
Asbestosis is an interstitial pneumonitis and fibrosis caused by inhalation of asbestos fibres after a latent period (1). Diagnosis requires radiological or histological evidence of structural pathology consistent with asbestos related disease, evidence of causation through occupational history, markers of disease such as pleural plaques and exclusion of other possible diagnoses (1). The role of bronchoalveloar lavage (BAL) in the assessment and diagnosis of interstitial lung disease remains controversial. Nonetheless, many studies have previously been performed to investigate whether BAL cellular profiles are useful in diagnosis, predicting outcome, measuring disease activity and even disease response to therapies. Indeed, BAL neutrophilia has been associated with disease severity and progression in IPF, hypersensitivity pneumonitis and sarcoidosis (2-8). BAL eosinophilia has also been associated with disease severity and outcome in IPF (9).
Also prior to the American Thoracic Society (ATS)/European Respiratory Society (ERS) reclassification of the idiopathic interstitial pneumonias, studies demonstrated that higher BAL fluid neutrophilia predicted a subsequent deterioration in pulmonary function test results (8).
We wished to investigate whether BAL was as useful in predicting the behavior of asbestosis with a UIP pattern on HRCT thorax.
Materials and methods
All patients with asbestosis with a UIP pattern diagnosed at the Birmingham Chest Clinic Occupational Lung Disease Unit between June 2000 and March 2012 were identified from local reports to the UK Surveillance of Work-Related and Occupational Respiratory Disease (SWORD) scheme. Each case was examined for the typical radiological appearance of UIP from radiology report along with a clinical diagnosis of asbestosis by an Occupational Lung Disease specialist physician [on the basis of clinical criteria, an unequivocal history of asbestos exposure and symptom latency as per recommendations (1)].
Results
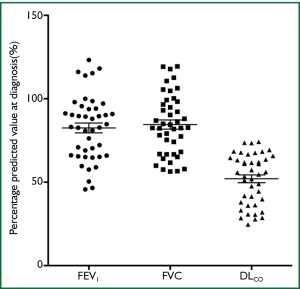
Data on 43 patients with significant asbestos exposure from various occupations, and findings of UIP radiologically were reviewed. All patients were symptomatic at diagnosis with the most common symptoms being breathlessness (95%) and cough (70%). 91% of patients had crackles on chest auscultation. All 43 patients had lung function data at diagnosis (FEV1, FVC, DLCO). Mean percentage predicted values (±SD) at diagnosis were as follows: FEV1 82.5% [19], FVC 84.5% [18], DLCO 52% [15] (Figure 1). All were male, mean age 74.5 (SD=7), mean asbestos exposure 16.3 years (SD=10), 21 patients had lung function data for at least one year after diagnosis. Mean annual decline in percentage predicted pulmonary function values (±SD) for the first year after diagnosis were as follows: FEV1 1.4% [7], FVC 2.1% [7], DLCO 4.2% [8]. 40 patients were ex s
mokers and 3 had never smoked.39 (91%) patients had benign asbestos pleural disease on radiological examination.
27 of total 43 patients were still attending our clinic for follow up. Since diagnosis 13 patients had died (5 ischaemic heart disease, 3 unknown, 2 community acquired pneumonia, 2 squamous cell lung cancer, 1 gastric adenocarcinoma)
21 patients had BAL performed as part of their work up at presentation.
3 patients had normal differential cell counts (lymphocyte ≤13%; neutrophil ≤3%; eosinophil ≤1%). 17 patients had raised neutrophil differentials (range, 6-80%); 6 patients had raised lymphocyte differential (range, 25-73%); 1 patient had raised eosinophil differential (5%). Median differential cell counts (with IQR) were as follows: neutrophils 14% [7-28], lymphocytes 5% [3-25], eosinophils 0% [0].
Correlation between BAL differential count and pulmonary function (Fev1. FVC and DLCO at diagnosis and their annual decline over 12 months) was analyzed using a Pearson correlation co-efficient, with 95% confidence interval.
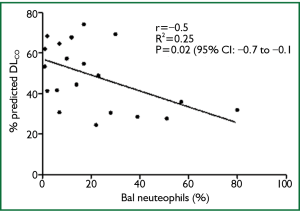
There was a significant inverse correlation between percentage BAL neutrophils and percentage predicted DLCO at diagnosis (n=21; P=0.02; r2= -0.25; CI, -0.77-0.08) (Figure 2), but not with DLCO decline over 1 year. There was no association between BAL lymphocytes or eosinophils and lung function at diagnosis and its decline over 12 months. These results were unrelated to smoking pack years. There was also no significant difference in the clinical characteristics and smoking history of those who had BAL and those who did not.
Discussion
The role of BAL in the diagnosis and work up of interstitial lung disease remains an area of considerable debate. What is clear is that if performed it should be to an exacting standard in centers that have expertise in processing the samples. Indeed the American Thoracic Society recently published: an Official American Thoracic Society Clinical Practice Guideline: the Clinical Utility of Bronchoalveolar Lavage Cellular Analysis in Interstitial Lung Disease, to help guide practice (10). While BAL cell differential has been shown to be predictive of disease progression in many forms of ILD including IPF, hypersensitivity pneumonitis and sarcoidosis, its role in asbestosis has had limited study. Neutrophil proportions in BAL have been shown to correlate with the amount of asbestos exposure (11) and with the severity of lung function impairment at diagnosis (12), however studies on disease progression are less clear. Rom et al. demonstrated that lung function decline had no relationship with the level of neutrophils in BAL yet Cullen et al. showed that the level of neutrophils in BAL was associated with progressive lung function deterioration (13,14). Our study’s finding are in keeping with those of Rom et al. and over all, indicate that like the other studies discussed, BAL neutrophilia is associated with impaired lung function at diagnosis only.
This study has a number of weaknesses including its small size and the fact that it is a retrospective study from a clinic-based population and is therefore subject to selection bias. Indeed there were no cases of asymptomatic asbestosis with significant CT findings in the sample. Cases were defined by a radiological diagnosis of UIP with an interstitial or occupational lung multi-disciplinary expert diagnosis of asbestosis; there were only three cases of biopsy-proven UIP (in patients with BAL lymphocytosis) although radiological diagnosis is sufficient where there is a classical appearance of UIP along with an appropriate history (8). This study also only comments on asbestosis with a UIP pattern and does not describe asbestosis associated with other interstitial radiology findings including intralobular reticulation, subpleural curvilinear lines, early subpleural dot-like opacities, interlobular septal thickening and ground-glass opacity, often features of early asbestosis. We also did not quantify asbestos bodies, a marker of asbestos exposure, a process that is particularly useful in those with an unconvincing history of asbestos exposure. This facility was not available to us at our institution at a standard recommended by the ERS Task Force Report on fibre analysis in biological samples (15). Fortunately our BAL patient group had a convincing history of exposure and virtually all had benign asbestos related pleural disease which has previously been found to correlate with fibre levels in the lung (15).
In conclusion, unlike in the case of IPF (which also has a UIP pattern), BAL neutrophilia is not predictive of decline in classic asbestosis with a UIP pattern and its routine use in this cohort of patients provides little if any additional benefit.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 2004;170:691-715. [PubMed]
- Haslam PL, Dewar A, Butchers P, et al. Mast cells, atypical lymphocytes, and neutrophils in bronchoalveolar lavage in extrinsic allergic alveolitis. Comparison with other interstitial lung diseases. Am Rev Respir Dis 1987;135:35-47. [PubMed]
- Drent M, van Velzen-Blad H, Diamant M, et al. Bronchoalveolar lavage in extrinsic allergic alveolitis: effect of time elapsed since antigen exposure. Eur Respir J 1993;6:1276-81. [PubMed]
- Lynch JP, Standiford TJ, Rolfe MW, et al. Neutrophilic alveolitis in idiopathic pulmonary fibrosis. The role of interleukin-8. Am Rev Respir Dis 1992;145:1433-9. [PubMed]
- Haslam PL, Turton CW, Lukoszek A, et al. Bronchoalveolar lavage fluid cell counts in cryptogenic fibrosing alveolitis and their relation to therapy. Thorax 1980;35:328-39. [PubMed]
- Rudd RM, Haslam PL, Turner-Warwick M. Cryptogenic fibrosing alveolitis. Relationships of pulmonary physiology and bronchoalveolar lavage to response to treatment and prognosis. Am Rev Respir Dis 1981;124:1-8. [PubMed]
- Watters LC, Schwarz MI, Cherniack RM, et al. Idiopathic pulmonary fibrosis. Pretreatment bronchoalveolar lavage cellular constituents and their relationships with lung histopathology and clinical response to therapy. Am Rev Respir Dis 1987;135:696-704. [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [PubMed]
- Peterson MW, Monick M, Hunninghake GW. Prognostic role of eosinophils in pulmonary fibrosis. Chest 1987;92:51-6. [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [PubMed]
- Robinson BW, Rose AH, James A, et al. Alveolitis of pulmonary asbestosis. Bronchoalveolar lavage studies in crocidolite- and chrysotile-exposed individuals. Chest 1986;90:396-402. [PubMed]
- Gellert AR, Langford JA, Winter RJ, et al. Asbestosis: assessment by bronchoalveolar lavage and measurement of pulmonary epithelial permeability. Thorax 1985;40:508-14. [PubMed]
- Rom WN. Accelerated loss of lung function and alveolitis in a longitudinal study of non-smoking individuals with occupational exposure to asbestos. Am J Ind Med 1992;21:835-44. [PubMed]
- Cullen MR, Merrill WW. Association between neutrophil concentration in bronchoalveolar lavage fluid and recent losses in diffusing capacity in men formerly exposed to asbestos. Chest 1992;102:682-7. [PubMed]
- De Vuyst P, Karjalainen A, Dumortier P, et al. Guidelines for mineral fibre analyses in biological samples: report of the ERS Working Group. European Respiratory Society. Eur Respir J 1998;11:1416-26. [PubMed]