Is left atrial appendage isolation a pyrrhic victory in the effort to treat atrial fibrillation?
Atrial fibrillation (AF) in the majority of people is a chronic disorder that results in progressive scarring and fibrosis of the atrium (1). Since AF often reflects a long-term vascular disease state driven by obesity, sleep apnea, hypertension, and diabetes, drivers of progressive fibrosis often persistent even after starting rhythm control therapies (2).
Pulmonary vein isolation remains the cornerstone of nonpharmacologic therapies for paroxysmal AF (3). With pulmonary vein isolation as a procedural endpoint, ablation of AF is successful in achieving sustained arrhythmia resolution in approximately 66% of patients (4,5). In the setting of arrhythmia recurrences, additional procedures can increase success rates to as high as 80% (6).
In patients with more advanced AF subtypes, such as persistent and longstanding persistent AF, the success rate of pulmonary vein isolation alone decreases to 40%, despite the use of additional ablation procedures (7). As experience with ablation grows, it has become apparent that these initial success rates are not maintained long-term; particularly in the setting of multiple cardiovascular comorbidities (8,9). The most recent consensus statement recommended that in patients with persistent AF, consideration of a more extensive ablation may be considered (3). Ironically, this recommendation to ablate more only adds more scarring to an already scarred atrium.
The art to AF ablation is treating enough of the atrium to lower risk of arrhythmia recurrence while not ablating too much that mechanical dysfunction or proarrhythmia occurs. As redo procedures are often required, inherently more of the atria are targeted. As we ablate more to ultimately conquer AF, we must ask ourselves, is this a Pyrrhic victory? Analogous to King Pyrrhus of Epirus, who in defeating the Roman armies in Heraclea and Asculum, lost so much in victory that he ultimately had to give up his campaign and return to Greece. With augmented ablation, sinus rhythm may prevail, but mechanical function can be lost resulting in long-term symptoms and consequences of atrial noncompliance and dysfunction (Figure 1) (10).
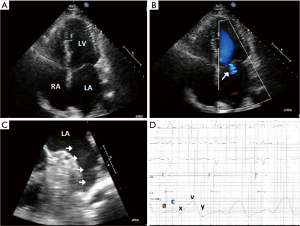
As we consider extrapulmonary vein sources of AF, 27% arise from the left atrial appendage (11). Both surgical and percutaneous left atrial appendage ligation systems have been shown to effectively isolate electrical activity of the appendage as a means to lower AF burden (12,13). In considering ablation within the appendage to target triggers, caution is required to avoid perforation or left phrenic nerve injury. To avoid ablation deep into the appendage, circumferential ablation is often performed to isolate it electrically from the atrium. However, with this approach mechanical dysfunction may develop thus increasing the stroke risk (14).
Three fundamental questions arise when considering electrical isolation of the left atrial appendage to improve long-term success after catheter ablation for persistent or longstanding persistent AF. First, can leave atrial appendage isolation be successfully performed with long-term durable results? Second, will it improve long-term success rates of the procedure? Third, if isolation can be successfully achieved, will the benefits outweigh the potential risks of stroke and stiff left atrial syndrome?
The BELIEF Trial (Effect of Empirical Left Atrial Appendage Isolation on Long-term Procedure Outcome in Patients with Persistent or Longstanding Persistent Atrial Fibrillation Undergoing Catheter Ablation) was a randomized study that compared two ablation strategies: standard ablation which was defined as pulmonary vein isolation with extra ablation as required versus standard ablation plus empirical left atrial appendage electrical isolation (15). At 12-month follow-up, 56% of patients with empiric left atrial appendage isolation compared to 28% with standard ablation alone were free of AF recurrence after a single procedure. The multivariate adjusted hazard ratio for arrhythmia risk recurrence of standard ablation without left atrial appendage isolation was 2.22 (95% CI: 1.29–3.81; P=0.004). In those patients that underwent empiric isolation of the appendage, and required a subsequent study, 63% had persistent isolation. In an observational study that examined the durability of left atrial appendage isolation after intentional or nonintentional isolation during catheter ablation, 73% had persistent isolation verified in a subsequent procedure (16). These two studies show that durable catheter based ablation to isolate the left atrial appendage can be successfully performed in the majority of patients. The BELIEF trial also found that this strategy was associated with improved procedural outcomes.
Next, regarding procedural safety, 62 patients that underwent empirical electrical left atrial appendage isolation at 6 months also had a transesophageal echocardiogram done to examine the appendage. In one patient with a subtherapeutic INR, an appendage clot was found. In 57% of the patients, impaired appendage function was demonstrated. However, as these patients were maintained on long-term anticoagulation therapy, no stroke or transient ischemic attacks were reported.
In contrast, a recent observational study of 50 patients that underwent left atrial appendage isolation at a median of 6.5 months, a left atrial appendage thrombus was seen in 21% (17). In addition, two patients experienced a stroke and one a transient ischemic attack who was not on anticoagulation. In this recent observational study, 42% of the patients were treated with a novel or direct oral anticoagulant rather than warfarin and 8% refused anticoagulation. Similar to the BELIEF study, long-term appendage dysfunction was common. In this study, average appendage flow was nearly 50% lower after isolation (0.2 vs. 0.5 m/s, P<0.01). A key difference between the two studies was choice on anticoagulation. Given the high rate of mechanical dysfunction of the appendage after isolation, long-term anticoagulation is critical. The second study highlighted that newer anticoagulants, with direct inhibition of a part of the clotting cascade, may not be a reliable substitute for the multiple levels of inhibition with warfarin. Neither study had sufficient follow-up to understand long-term consequences of a potentially stiffer left atrium. Both studies highlighted a significant potential risk of stroke and that this procedure alone makes long-term anticoagulation with warfarin requisite. If warfarin is not tolerated or a patient is at high risk for bleeding complications, early evidence suggests potential benefit of left atrial appendage occlusion after electrical isolation (18).
The BELIEF study suggests that left atrial appendage isolation is not merely a Pyrrhic victory in our efforts to treat AF. However, appendage isolation is a victory with significant risks and consequences and needs to be performed with these potential risks in mind and by those with the greatest experience with ablation in a stepwise manner. Most importantly, the procedure should only be performed when long-term uninterrupted anticoagulation can be used or a plan of left atrial appendage occlusion is possible. In cases that warfarin may have to be discontinued, and left atrial appendage occlusion is not available or possible, consider thoracoscopic left atrial appendage removal. Regarding the long-term impact of left atrial appendage isolation on the function of the left atrium, data are still lacking. As stiff left atrial syndrome can lead to debilitating symptoms, even in patients that maintain sinus rhythm, this should prompt caution in the majority of physicians that perform AF ablation. Caution should be advised regarding left atrial appendage isolation until long-term outcomes are available.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dzeshka MS, Lip GY, Snezhitskiy V, et al. Cardiac Fibrosis in Patients With Atrial Fibrillation: Mechanisms and Clinical Implications. J Am Coll Cardiol 2015;66:943-59. [Crossref]
- Bunch TJ, May HT. Atrial fibrillation: a risk factor or risk marker? Eur Heart J 2016;37:2890-2. [Crossref]
- Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 2012;9:632-696.e21. [Crossref]
- Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333-40. [Crossref]
- Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J 2006;27:216-21. [Crossref]
- Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549. [Crossref]
- Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of long-standing persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010;7:835-46. [Crossref]
- Bunch TJ, May HT, Bair TL, et al. Five-year outcomes of catheter ablation in patients with atrial fibrillation and left ventricular systolic dysfunction. J Cardiovasc Electrophysiol 2015;26:363-70. [Crossref]
- Jacobs V, May HT, Bair TL, et al. The impact of risk score (CHADS2 versus CHA2DS2-VASc) on long-term outcomes after atrial fibrillation ablation. Heart Rhythm 2015;12:681-6. [Crossref]
- Gibson DN, Di Biase L, Mohanty P, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 2011;8:1364-71. [Crossref]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation 2010;122:109-18. [Crossref]
- Benussi S, Mazzone P, Maccabelli G, et al. Thoracoscopic appendage exclusion with an atriclip device as a solo treatment for focal atrial tachycardia. Circulation 2011;123:1575-8. [Crossref]
- Afzal MR, Kanmanthareddy A, Earnest M, et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm 2015;12:52-9. [Crossref]
- Kim D, Shim CY, Hong GR, et al. Clinical Implications and Determinants of Left Atrial Mechanical Dysfunction in Patients With Stroke. Stroke 2016;47:1444-51. [Crossref]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J Am Coll Cardiol 2016;68:1929-40. [Crossref]
- Reissmann B, Rillig A, Wissner E, et al. Durability of wide-area left atrial appendage isolation: Results from extensive catheter ablation for treatment of persistent atrial fibrillation. Heart Rhythm. 2016. [Epub ahead of print]. [Crossref]
- Rillig A, Tilz RR, Lin T, et al. Unexpectedly High Incidence of Stroke and Left Atrial Appendage Thrombus Formation After Electrical Isolation of the Left Atrial Appendage for the Treatment of Atrial Tachyarrhythmias. Circ Arrhythm Electrophysiol 2016;9:e003461. [Crossref]
- Panikker S, Jarman JW, Virmani R, et al. Left Atrial Appendage Electrical Isolation and Concomitant Device Occlusion to Treat Persistent Atrial Fibrillation: A First-in-Human Safety, Feasibility, and Efficacy Study. Circ Arrhythm Electrophysiol 2016;9:e003710. [Crossref]


