Practice patterns in venous thromboembolism (VTE) prophylaxis in thoracic surgery: a comprehensive Canadian Delphi survey
Introduction
The reported incidence of venous thromboembolic events (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), following lung resection varies. The literature reports a wide range of incidence, from 5–15.2% (1-3). This variation in rate is due to differences in the method of detection (routine post-operative screening versus diagnosis of symptomatic patients only), type of post-operative prophylaxis used (mechanical and/or pharmacological), and thromboprophylaxis initiation/duration. Currently, the American College of Chest Physicians (ACCP) 9th edition guidelines recommend the use of in-hospital routine VTE prophylaxis with either low-dose unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) for the post-operative thoracic surgery population (grade 1B evidence) (4). Of note, the guidelines provide no specification on the duration of thromboprophylaxis, nor the role of extended out-of-hospital prophylaxis. A screening study aimed at detecting both symptomatic and sub-clinical post-lung resection VTE reported a markedly higher than expected incidence of PE at 14%, despite following current ACCP guidelines (5). Our group recently completed a cohort study prospectively screening post lung resection patients, and found a similarly high incidence of 12.1% (6), demonstrating the significant burden of VTE in this population.
Recent evidence suggests that, in the case of lung cancer surgery, up to 23% of post-operative thrombotic events occur in the post-discharge period (7). Similarly, Mason et al. demonstrated that in a cohort of patients undergoing pneumonectomy for cancer, the peak incidence of VTE incidence occurred 7 days after surgery, a point at which most patients had already been discharged from hospital (8). The delayed incidence of post-oncologic surgery VTE is supported by a large prospective observational trial of 2,373 cancer patients, where 40% of events occurred greater than 21 days after the date of the index surgery (9). The use of extended out-of-hospital prophylaxis is an established practice in other surgical disciplines such as high-risk orthopedic and major oncologic abdominal surgeries (4,7,10). Considering this practice and the high risk of VTE, prolonged VTE prophylaxis in the thoracic surgery population may offer similar benefits to those seen in other surgical specialties (4).
Limited guidelines addressing post-thoracic surgery VTE prophylaxis have presumably resulted in a substantial variability between centres and practitioners, with regards to type of pharmacological agents used, role of mechanical devices, timing of prophylaxis initiation, determination of high-risk patient subgroups, and the indications for and benefit of prolonged prophylaxis. The objectives of this study were to describe current practice patterns amongst practitioners who treat thoracic surgery patients, establish a Canadian national consensus on the approach to VTE prophylaxis initiation and duration in the thoracic surgery community, and to determine which high risk subgroups are perceived to potentially benefit from extended thromboprophylaxis.
Methods
A modified Delphi survey was used to establish national trends and standards (11). A series of three anonymous iterative online surveys were distributed to eligible Canadian participants, which included thoracic surgeons, thoracic anesthesiologists and thrombosis physicians (including hematologists and internists) across Canada. A population-based sampling frame was used for this cross-sectional study. Initial surveys were disseminated using the Canadian Association of Thoracic Surgery (CATS) registry to a total of 84 thoracic surgeons. The selection of anesthesiologists and thrombosis physicians was based on the opinion of an expert panel of leaders who have participated in the development of postoperative guidelines. The inclusion of anaesthesiologists was deemed important given the interplay between VTE prophylaxis and the use of epidurals for post-operative pain management. Authors did not participate in survey responses. The first round of survey distribution occurred in August 2014, with rounds 2 and 3 taking place in November 2014 and January 2015, respectively. For each round, a total of duration of 30 days was allowed to elapse before the deadline to response submission. Survey dissemination and response was completed electronically using LimeSurvey software, with two reminder emails circulated for each iteration.
Surveys consisted of a series of questions addressing different parameters of importance in thoracic surgery thromboprophylaxis. These were categorized into four groups: (I) perioperative risk factors for VTE; (II) the impact of those factors on selecting extended prophylaxis; (III) the type and method of preferred prophylaxis (pharmacological and/or mechanical); and (IV) the timing of treatment initiation and duration. A steering committee consisting of a hematologist, a thoracic surgeon and a research coordinator created and modified the list of parameters included in the survey. Following the first round, the survey questions were re-examined in order to eliminate any parameters that were deemed repetitive or lacking clinical relevance. Participants were asked to rate each parameter on a 10-point scale based on magnitude of importance. Results of each round were summarized using descriptive statistics, and a de-identified summary was circulated to participants along with the next survey round. In accordance with Delphi methodology, respondents were encouraged to consider the collective results of their colleagues in the previous round when answering. Figure 1 summarizes the framework used to complete the outlined Delphi process. The distribution of scores for each parameter was measured and a mean score calculated in order to assess variance. Agreement was defined a priori as an item demonstrating a coefficient of variance (Cv) ≤0.3. This cut-off was determined based on established literature, in order to indicate reasonable and robust internal agreement (11-13). Once a survey item was deemed to reach this threshold on two consecutive rounds, it was dropped from future rounds. In each round, a secondary sub-stratified analysis of within-specialty discordance was measured in order to examine whether any apparent lack of agreement was confounded by specialty. It is important to note that the study was not powered for this type of secondary analysis. All data was collected prospectively and analyzed using SPSS statistical software program, version 20.0 for Windows (SPSS, Chicago, IL, USA). Ethics review was not required by our institution, and implied consent was assumed by voluntary response.
Results
The first iteration of surveys had a total of 72 respondents, and overall represented the greatest proportion of participants among all three rounds (85.7% response rate). Rounds 2 and 3 consisted of 57 and 50 respondents respectively. The Delphi questionnaire process was halted after round 3 as it appeared unlikely that further iterations would lead to consensus. In rounds 1 and 2, the distribution among specialities was initially skewed towards thoracic surgeons compared to thrombosis physicians and anesthesiologists (41% vs. 32% and 24%; as well as 54% vs. 21% and 16% respectively). The 3rd round, however, demonstrated an even distribution among the 50 respondents with Surgeons representing 36% of respondents, while thrombosis physicians and anesthesiologists represented 36% and 28% respectively. Table 1 provides a detailed breakdown of participant demographics by round and specialty. Taken together, 30.6% self-reported specific expertise in post-thoracic surgery VTE prophylaxis (Table 2). Analysis of round 1 respondents, comprising the overall study sample size, demonstrated that participants were in independent practice for an average of 13 years (range: 1–35), and 66.7% were actively prescribing prophylactic agents for thoracic patients.

Full table

Full table
Current practice patterns
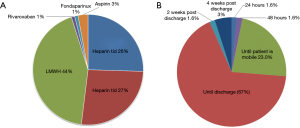
Analysis of current trends in practice demonstrated that 44% of respondents used LMWH as the pharmaceutical agent of choice. In contrast, 26% and 27% used UFH twice-per-day (BID) and three-times-daily (TID), respectively. Less than 5% of respondents reported the use of oral agents such as rivaroxaban or aspirin (Figure 2A). The initiation of prophylaxis varied substantially, with 30% reporting the initial dose given pre-operatively prior to patient entry in the operating room, 34% during anesthetic induction, and 36% immediately post-operatively. Approximately 67% of participants reported a preference for prophylaxis duration up to the time of discharge, with 23% of respondents halting pharmacological prophylaxis once the patient is mobile, a practice not in concordance with any of the current guidelines. Only 1.6% and 3% of respondents reported the use of extended prophylaxis up to 2 and 4 weeks post-discharge, respectively (Figure 2B). Among practitioners employing mechanical prophylactic devices, 66% of participants reported the use of sequential compression devices (SCD), while the remainder used TED compression stockings.
Perioperative risk perception
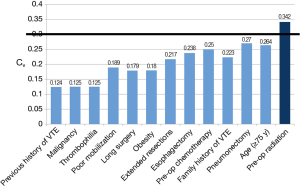
A complete list of the perioperative factors assessed as determinants of VTE risk is provided in Figure 3. All of these factors, with the exception of pre-operative radiation (mean =5.32, Cv =0.34), fell under the pre-defined threshold. Agreement among respondents was accordingly reached by round 2 for all such parameters. Subgroup analysis by specialty depicted comparable values for most of the factors assessed. In contrast, disagreement on pre-operative radiation as a risk factor was largely attributable to thoracic surgeon responses (Cv =0.40), whereas the other specialties reported values below the threshold of accepted variance.
Impact on selecting extended prophylaxis
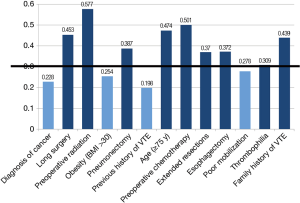
Among the peri-operative factors deemed to be important determinants of post-operative VTE risk, agreement appeared to exist in the following parameters, thus establishing them as having an impact on the respondents’ decision to use extended VTE prophylaxis: history of previous DVT/PE (mean =8.52, Cv =0.198), diagnosis of cancer (mean =7.88, Cv =0.228), obesity (mean =6.77, Cv =0.254) and poor mobilization (mean =6.96, Cv =0.278). For these risk factors, agreement was reached by the second round (Figure 4). The third iteration did not provide any added consensus for the remaining parameters. Thrombophilia was reported as having a perceived high impact on the decision to provide patients with extended prophylaxis, with the final results just exceeding the pre-set threshold (mean =7.82, Cv =0.309). Despite the agreement on deterministic factors indicating the need for extended prophylaxis, respondents demonstrated no agreement on routine use of out-of-hospital prophylaxis.
Type and method of prophylaxis
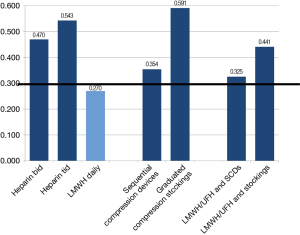
Variables pertaining to both pharmacological and mechanical means of thromboprophylaxis were assessed in this category. The only item that reached agreement by the third iteration was the use of LMWH post-operatively (mean =8.00, Cv =0.270). No agreement was reached regarding the use of other pharmacological agents, mechanical prophylaxis or the preference for combination therapies (Figure 5). A large difference in mean scores existed between thoracic surgeons and thrombosis physicians. LMWH/UFH with the addition of SCD had a mean response of 7.79 in the surgeon group and 5.83 in the thrombosis physicians. Similarly, LMWH/UFH and graduated compression stockings had a mean score of 6.00 for surgeons and 3.92 for thrombosis physicians. Thrombosis physicians demonstrated the least agreement and the lowest preference for mechanical prophylaxis although the results were highly variable. Thoracic surgeons demonstrated intermediate consensus and great variability of results, with an overall preference for SCD. Anesthesiolgists had the greatest agreement and depicted a preference for SCD over graduated compression stockings with and without medical prophylaxis.
Timing of prophylaxis initiation and duration
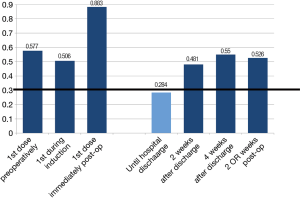
The only temporal parameter that depicted agreement among all respondents was the administration of pharmacological prophylaxis until hospital discharge. This was reached at the second iteration, and applied to both UFH (mean =8.08, Cv =0.276) or LMWH (mean =8.09, Cv =0.284) (Figure 6). No notable differences pertaining to timing and initiation existed between the use of LMWH or UFH. Relative to other specialties, thoracic surgeons demonstrated the highest degree of agreement with preferences for prophylaxis until hospital discharge. No agreement on the timing (pre-, intra- or post-operatively) of thromboprophylaxis initiation was reached. More importantly, there was no agreement as to the use of extended prophylaxis past the point of hospital discharge (mean =5.73, Cv =0.526 for LMWH; and mean =5.05, Cv =0.626 for UFH). This result was consistent for both 2- and 4-week post-discharge, and represented each of the specialties alone, as well as all groups combined.
Discussion
To date, this is the first attempt to systematically capture the current practice patterns and opinions regarding venous thromboembolic prophylaxis following thoracic surgery in Canada. The sample of respondents in this study consisted of highly qualified clinicians with experience in treating post-thoracic surgery patients. With regionalization of care, the Canadian thoracic surgery community is relatively small and most surgeons work in academically affiliated hospitals. Accordingly, despite the relatively small number of respondents, CATS affords a unique opportunity to assess practice patterns at a national level with reasonable comprehensiveness.
In general, respondents demonstrated strong agreement in identifying risk factors for VTE, and which of those factors may potentially influence the decision for extended post-hospital discharge prophylaxis. There was limited agreement however on the type of prophylaxis (pharmacological, mechanical and/or both), as well as the initiation and duration of thromboprophylaxis—indicating a high degree of variability in the delivery of thromboprophylaxis in this population. The only reliable factor of agreement was the use of LMWH for the duration of post-operative hospital stay until discharge.
This practice variability has been recently affirmed by a similar online survey out of the University of Kentucky, attempting to assess post-esophagectomy VTE prophylaxis practice patterns amongst 77 thoracic surgeons (14). These results demonstrated that opinions and practices varied widely, with up to 30% of participants using suboptimal dosing. Interestingly, while 34% of surgeons estimated that more than a fifth of the post-esophagectomy VTE occur post-discharge, only 13% routinely discharged patients home with pharmacoprophylaxis. Respondents concluded that much of the variety in clinical practice was attributed to a lack of specific guidelines and evidence. Our study echoed these findings, but had the added benefit of including a greater range of respondents across specialties other than thoracic surgeons, and utilized more rigorous variation analysis to establish agreement across disciplines. In contrast to the single round descriptive review presented by Zwischenberger et al. (14), we used an iterative process over three rounds of surveys, allowing for a more comprehensive assessment of within and between specialty consensuses or disagreements.
The most comprehensive analysis to-date pertaining to post-thoracic surgery VTE prophylaxis, a 2015 Cochrane review assessing the effects of primary thromboprophylaxis on the incidence of symptomatic VTE and major bleeding in patients undergoing cardiac and thoracic surgery, determined that evidence regarding the efficacy and safety of this practice is lacking (15). None of the studies assessed the effects of mechanical prophylaxis on post-operative VTE incidence, and there was no reported statistically significant difference between pharmacological prophylaxis in terms of symptomatic VTE or major bleeding. Methodological assessment confirmed that all included studies were graded as low to very low quality. As such, it was determined that no conclusion could be drawn concerning the benefit-to-risk balance, and that clinical decisions should still be made on a case-by-case basis. Despite these technical limitations, the review analysed seven studies pertinent to thoracic surgery, with an overall incidence of symptomatic VTE of 0.52% (15/2,890) (15).
Another recent systemic review by Christensen et al. summarizing the results of 19 studies reported an overall risk of clinically detected VTE in patients undergoing operations for primary lung cancer to be as low as 2.0%, with the highest incidence present within 1 month of the operation (16). Despite analysing data from over 10,660 patients, all but one of the included studies were retrospective with marked heterogeneity, limiting the ability to pool the data. The VTE estimates reported in these reviews are in stark contrast to more recent literature demonstrating a higher incidence rate (7,17,18). Using computed tomography (CT) pulmonary angiogram and lower extremity venous Doppler ultrasound screening, we recently found that the prevalence of post-lung cancer resection VTE was 12.1%, and that over 75% patients were asymptomatic (6), highlighting the potential under-detection of this phenomenon.
Thoracic surgery patients present a unique subset of cancer patients, with arguably greater risk of thromboembolic complications. Many patients have advanced malignancies, additional comorbidities, and extensive resections with a high risk of complications, requirement for hospitalization and bed rest. The most relevant recommendations for thoracic surgery VTE thromboprophylaxis are provided in the 9th edition ACCP guidelines. These were determined based on only two old prospective trials (2,3) and several other low quality retrospective publications (4). This demonstrates the lack of quality studies available for guideline formation, and highlights the need for comprehensive research to determine the efficacy and safety of prophylaxis in this patient population.
The clinical burden of post-operative VTE following thoracic surgery is substantial, and the risk continues to increase past the point of hospital discharge (7,15). With the effectiveness of in-hospital prophylaxis already established (4), there is evidence to support extended post-discharge prophylaxis recommendations following orthopedic and major pelvic surgery. It is likely that the thoracic surgery population would benefit from guidelines similar to those of orthopedic and general surgery. This national Delphi survey demonstrates that the majority of experts utilize some method of prophylaxis, and are cognisant of heightened risk profiles that may necessitate extended prophylaxis. However, specific guidelines are lacking. As such, this study serves as a benchmark for current Canadian practice, but more importantly, it supports the need for scientifically rigorous research to formulate recommendations.
The strengths of this study are its comprehensive nature and robust methodology used to establish national perspectives and agreement across different parameters. We used an iterative process, and sought responses from relevant opinion holders from various disciplines involved in peri- and post-thoracic surgery patient care. Thus, the data reflects opinions from multiple viewpoints, and is more representative of the contemporary clinical practice in Canada albeit with several limitations. The use of survey data predisposes to reporting bias, given that all information was self-reported. Also, despite the inclusion of multiple specialists across disciplines, there was a discrepancy in the distribution of respondents, particularly in the first and second rounds of iteration. This may skew the data in favour of those specialities that were more represented and limit the external validity of the study. In addition, the possibility of selection bias may limit generalizability of the results, given that respondents (particularly anesthesiologists and thrombosis experts) were more likely to have strong academic affiliations with marked expertise. As such their responses may not reflect typical non-academic practice patterns. Finally, the lack of free-text responses might have prohibited respondents from adding relevant information that they could have deemed clinically important. Within those limitations, however, we believe the results of this analysis will help to design future studies.
Conclusions
Our findings suggest that there is no consensus on VTE prophylaxis regimens following thoracic surgery in Canada. Agreement does, however, exist regarding the risk factors that heighten patient risk including after hospital discharge. Higher quality research is needed to facilitate the development of VTE prophylaxis guidelines that can be used to define appropriate care standards.
Acknowledgements
None.
Footnote
Conflicts of Interest: Moderated poster presentation at the 25th International Society on Thrombosis and Haemostasis Congress/61st Annual Scientific and Standardization Committee Meeting; Toronto, Ontario, Canada; June 20–25 2015.
Ethical Statement: Given the survey nature of this study, ethics approval was not required by our institution. All data collection and results presentation are anonymous and confidential.
References
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975-80. [Crossref]
- Cade JF, Clegg EA, Westlake GW. Prophylaxis of venous thrombosis after major thoracic surgery. Aust N Z J Surg 1983;53:301-4. [Crossref]
- Azorin JF, Regnard JF, Dahan M, et al. Efficacy and tolerability of fraxiparine in the prevention of thromboembolic complications in oncologic thoracic surgery. Ann Cardiol Angeiol (Paris) 1997;46:341-7.
- Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e227S-77S.
- Daddi G, Milillo G, Lupattelli L, et al. Postoperative pulmonary embolism detected with multislice computed tomography in lung surgery for cancer. J Thorac Cardiovasc Surg 2006;132:197-8. [Crossref]
- Agzarian J, Hanna WC, Schneider L, et al. Postdischarge venous thromboembolic complications following pulmonary oncologic resection: An underdetected problem. J Thorac Cardiovasc Surg 2016;151:992-9. [Crossref]
- Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg 2011;254:131-7. [Crossref]
- Mason DP, Quader MA, Blackstone EH, et al. Thromboembolism after pneumonectomy for malignancy: an independent marker of poor outcome. J Thorac Cardiovasc Surg 2006;131:711-8. [Crossref]
- Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006;243:89-95. [Crossref]
- Kakkar VV, Balibrea JL, Martínez-González J, et al. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost 2010;8:1223-9. [Crossref]
- Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376-80. [Crossref]
- Von Der Gracht HA. Consensus measurement in Delphi studies: Review and implications for future quality assurance. Technol Forecast Soc 2012;79:1525-36. [Crossref]
- English GM, Kearnan GL. The prediction of air travel and aircraft technology to the year 2000 using the Delphi method. Transp Res 1976;10:1-8. [Crossref]
- Zwischenberger BA, Tzeng CW, Ward ND, et al. Venous Thromboembolism Prophylaxis For Esophagectomy: A Survey of Practice Patterns Among Thoracic Surgeons. Ann Thorac Surg 2016;101:489-94. [Crossref]
- Di Nisio M, Peinemann F, Porreca E, et al. Primary prophylaxis for venous thromboembolism in patients undergoing cardiac or thoracic surgery. Cochrane Database Syst Rev 2015;6:CD009658.
- Christensen TD, Vad H, Pederson S, et al. Venous thromboembolism in patients undergoing operations for lung cancer: A systematic review. Ann Thoracic Surg 2014;97:394-400. [Crossref]
- Raja S, Blackstone EH, Murthy SC. Caught between a rock and a hard place: Venous thromboembolism screening in high-risk patients. J Thorac Cardiovasc Surg 2016;151:1000-1. [Crossref]
- Hachey KJ, Hewes PD, Porter LP, et al. Caprini venous thromboembolism risk assessment permits selection for postdischarge prophylactic anticoagulation in patients with resectable lung cancer. J Thorac Cardiovasc Surg 2016;151:37-44.e1. [Crossref]