Differential diagnosis of acute miliary pulmonary tuberculosis from widespread-metastatic cancer for postoperative lung cancer patients: two cases
Introduction
Lung cancer is the most common incident cancer and the leading cause of cancer-related death worldwide (1). Accurate diagnosis is of great importance and directly influences the following treatment. Radiology is the main tool used for diagnosis and staging of lung cancer clinically. However, pulmonary infections occasionally present as inflammatory pseudotumour of lung. In such case, differential diagnosis seems difficult through radiographic findings and clinical manifestation. Diversity of infectious diseases like bacteria (2), mycobacteria (3,4), fungi (5,6) and viruses (7) cause inflammatory lung lesions and resemble pulmonary carcinoma, which make definite diagnosis difficult. General histologic findings in 2908 patients with a presumed diagnosis of pulmonary neoplasm were found to be fungal infections (46%, including histoplasmosis, cryptococcosis, coccidiomycosis), mycobacteriosis (27%), bacteriosis (22%) and parasitic lesions (5%, dirofilariasis) that mimicked lung cancer (8). Besides that, it is equally important to differ secondary lung cancer from primary lung cancer. It is reported that secondary lung cancer accounts for 8.6 percent of all the 659 resected lung cancer cases (9).
Here we report two cases of postoperative patients with lung adenocarcinoma who developed diffuse nodules bilaterally in a month and needed to be distinguished between malignancies and infectious diseases. Although there is much resemblance of the two cases in disease process, imaging characters and even laboratory examination results, different results were obtained; patient in case one was finally diagnosed as acute miliary pulmonary tuberculosis (TB) by positive sputum culture while patient in case two was considered as relapse of cancer by fiberoptic bronchoscopy.
Case presentation
Case one
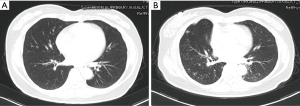
A 45-year-old woman was admitted to the department of thoracic surgery for the nodule on the right-middle lobe of lung found on chest contrast-enhanced computed tomography (CT) scan, which enlarged to 1cm (the maximum diameter) from 0.7 cm (Figure 1A,B) in 6 months. Considering a malignant tumor, the patient underwent a right-middle lobectomy and lymph node dissection by video-assisted thoracic surgery (VATS) after signing informed consent forms. During operation, it was found that visceral pleura was invaded by the tumor. Adenocarcinoma was confirmed by hematoxylin and eosin (H&E) staining of the tissue. The cancer was classified as T2aN0M0 (stage Ib) according to AJCC staging system. Further genetic analyses detected EGFR mutation (exon 21 point mutation L858R).
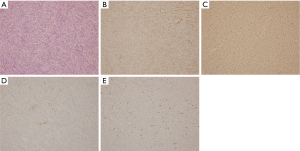
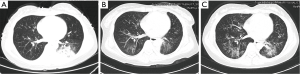
One month after the surgery, the patient complained a severe cough in outpatient department. A series of investigations were conducted for diagnosis. The thoracic CT scan found that the right-middle lung was absent (Figure 1C) and compared with preoperative image (Figure 2A), numerous miliary nodules with blurred border and inhomogeneous density distributed diffusely in both lungs (Figure 2B). The laboratory examinations, such as full blood count and biochemical analyses, were normal except for raised ESR (erythrocyte sedimentation rate) and mildly elevated CA-125. Her temperature was normal too. After reviewing the history of the patient, we got to know that she was diagnosed with left inguinal dermatofibrosarcoma protuberans (DFSP) 7 years ago and thereafter received tumor resection twice and then an extended resection plus dermatoplasty after recurrence. The H&E staining (Figure 3A) showed fairly uniform spindle cells located mainly in the dermis. The immunohistochemical (IHC) staining revealed that the mass presented strongly positive reaction for CD34 (Figure 3B) and vimentin (Figure 3C), low expression of CD10 (Figure 3D) and Ki-67 (Figure 3E).
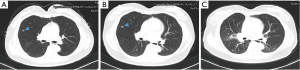
Pulmonary TB, fungal infection, human cytomegalovirus (HCMV) infection, recurrent cancer and secondary lung cancer were taken into consideration. After a multidisciplinary team meeting, pulmonary infection especially TB was the most likely diagnosis. Further examinations were done to distinguish these diseases. C-reaction protein (CRP), procalcitonin (PCT), HCMV DNA detected by real-time fluorescence PCR and fungal 1,3-β-D-glucan were negative. The positive outcomes of TB-IGRA (interferon-gamma release assays) and mycobacterium TB culture of sputum confirmed the diagnosis of pulmonary TB. The symptom and pulmonary foci (Figure 4A,B) improved markedly after the immediate chemotherapy for mycobacterium TB.
Case two
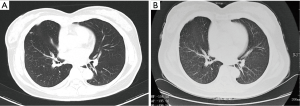
A 42-year-old female patient presented to the outpatient department with the compliant of repeated cough and fever. Then she underwent a thoracic CT scan and was diagnosed as lobar pneumonia for the consolidation presented at the inferior lobe of left lung, but without the enlargement of hilar and mediastinal lymph nodes (Figure 5A). However, the pulmonary lesion did not shrink at all after she received antibiotic therapy. CT-guided transthoracic needle biopsy was performed and she was pathologically diagnosed as lung adenocarcinoma. Then she suffered left lower lobectomy and lymph node dissection by VATS. The postoperative staging was stage IIB (pT3N0M0). One month later, a routine chest CT scan was performed and the images indicated widely scattered nodules, ground glass opacity and patchy shadows in bilateral lungs (Figure 5B). The radiologist thought that the nature of the nodules was unclear and it may co-exist with pulmonary infection including the possibility of fungal infection. However, except for CA-125 increasing by 2.3 times, other tumor biomarkers (CEA, CYPFA21-1 and NSE) and the white blood cell (WBC) count were all in normal range. Further biochemical analyses including acid-fast bacilli staining of sputum smear for mycobacteria and sputum culture for bacteria and fungi were all negative as well. Finally, cytological examination by fiberoptic bronchoscope brushing and bronchoalveolar lavage (BAL) were carried out and some adenocarcinoma cells were found in right upper lobe but no mycobacteria and fungi were detected in BAL liquid. Then the patient received chemotherapy with a regimen of cisplatin-pemetrexed disodium. But the disease progressed (Figure 5C) after three cycles of chemotherapy, and she rejected a second-line therapy and asked for discharge at last.
Discussion
Surgery, chemotherapy and radiotherapy are the most common treatment approaches of lung cancer which are far more different from that of infection diseases. The possibility of surgery is greatly depending on the nature of cancer—primary or secondary. Although pathologic diagnosis is the golden standard, biopsy sometimes is not suitable for all patients. Therefore, it is hard to differentiate primary lung cancer from other diseases through radiological assessments because radiographic findings and clinical manifestations often suggest tumor. Furthermore, previous studies have shown that pulmonary carcinoma can coexist with bacillosis, TB or mycosis (10,11). The complexity of disease often delays appropriate therapy. Here we report two cases that the patients developed wide-spread miliary lesions in both lungs one month postoperatively.
Timely differential diagnosis is needed. For most oncologists, disease relapse is a major concern when encountered similar situation as the two cases. Matthews et al. reported that 35% of the patents who were thought to receive a curative surgical procedure would suffer from local spread or distant metastases within one month (12). In case one, metastatic disease is not considered firstly because it is a very early stage (stage Ib) disease. But in case two, the possibility of cancer relapse is much higher than that in case one because the disease is classified as pathologic T3. An interesting thing is that they got the same laboratory examination results: tumor biomarkers (CEA, CYFRA21-1, NSE) were normal except for the elevated CA125. Although there is striking resemblance of the two cases, two completely different results were obtained; finally, patient in case one was diagnosed as acute miliary pulmonary TB by positive sputum culture while patient in case two was diagnosed as metastatic disease by fiberoptic bronchoscopy.
It is reported that pulmonary TB (13), fungal infection (6) and HCMV infection (7) mimic lung cancer which is sometimes indistinguishable from each other by radiologic tools like CT and positron emission tomography (PET) (14). In case one, the clinical manifestation and radiological images were in accord with the characteristics of the pulmonary infections. But bacterial pneumonia was out of the consideration because the patient’s temperature and complete blood count were in normal range. Further investigations were done for accurate diagnosis. Just as our speculation, positive outcomes of TB-IGRA and mycobacterium TB culture of sputum confirmed the diagnosis of pulmonary TB and negative results of HCMV DNA by real-time fluorescence PCR and fungal 1,3-β-D-glucan denied HCMV and fungal infection. The markedly improved symptom and imaging findings after anti-TB treatment reinforced this argument. However, why the patient developed an acute miliary TB (Figure 2B) one month after the surgery remains unknown and no obvious foci was observed preoperatively (Figure 2A). Furthermore, the possibility of pre-existed or undiscovered TB can’t be ruled out and surgery may be a precipitating factor for the dissemination of mycobacterium TB. Huang et al. reported that TB developed in 2% (44/2,177) of gastrectomy patients with malignancy but without history of TB, indicating that it is easier to develop TB as a result of suppressed immune system caused by surgery or cancers (15). Before anti-TB treatment, a fiberoptic bronchoscopy should be carried out because it is crucial for the diagnosis of both complicated infection and lung cancer (16-18). But the patient refused. Hence, we cannot make sure whether she had a bronchial TB. However, as the patient did not have a definite TB lesion before surgery, the positive sputum culture of mycobacterium TB can be an indirect evidence for bronchial TB. In case two, there are many similarities to case one not only the disease process but also the examination results. Infection of bacteria and fungi was ruled out by negative sputum culture. But mycobacteria cannot be excluded completely because the positive rate of smear for acid-fast bacilli is very low. The patient was diagnosed as metastatic disease finally as adenocarcinoma cells were found in the bronchia of contralateral lung while no mycobacteria or fungi were detected in BAL liquid.
Furthermore, in case one the history of left inguinal DFSP increased the difficulty of differential diagnosis. DFSP is a rare cutaneous malignancy that originates from the dermis and accounts for less than 2% of all soft tissue sarcomas (19). CD34 and vimentin are expressed nearly in all DFSP and CD10 is partially positive (20). Here are the reasons why we think that there is low possibility of lung cancer secondary to DFSP for this patient. First, the probability of regional or distant metastasis is less than 4% of all cases, although it is more common after recurrence and with high-grade lesions (21,22). Next the lung metastases often distributes in the peripheral and subpleural lung regions. At last, the long course of disease history (7 years) and very low Ki-67 index (<5%) indicates the low proliferation rate which suggest the inert tumor nature and low metastatic possibility.
Conclusions
Diffuse nodules of lungs developed postoperatively should be carefully distinguished between metastatic cancer and infectious diseases. Accurate diagnosis directly influences the following treatment scheme and disease course.
Acknowledgements
The authors would like to thank Dr Hongying Zhang et al., Department of Pathology, West China Hospital, Medical School, Sichuan University, for assistance and advice on the histopathological diagnosis.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Madhusudhan KS, Gamanagatti S, Seith A, et al. Pulmonary infections mimicking cancer: report of four cases. Singapore Med J 2007;48:e327-31. [PubMed]
- Schweigert M, Dubecz A, Beron M, et al. Pulmonary infections imitating lung cancer: clinical presentation and therapeutical approach. Ir J Med Sci 2013;182:73-80. [Crossref] [PubMed]
- Van Zwanenberg D, Barry M. A case of sarcoidosis and three cases of atypical tuberculosis in a family. Lancet 1955;268:483-5. [Crossref] [PubMed]
- Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest 2002;121:1988-99. [Crossref] [PubMed]
- Chung CR, Lee YC, Rhee YK, et al. Pulmonary coccidioidomycosis with peritoneal involvement mimicking lung cancer with peritoneal carcinomatosis. Am J Respir Crit Care Med 2011;183:135-6. [Crossref] [PubMed]
- Karakelides H, Aubry MC, Ryu JH. Cytomegalovirus pneumonia mimicking lung cancer in an immunocompetent host. Mayo Clin Proc 2003;78:488-90. [Crossref] [PubMed]
- Rolston KV, Rodriguez S, Dholakia N, et al. Pulmonary infections mimicking cancer: a retrospective, three-year review. Support Care Cancer 1997;5:90-3. [Crossref] [PubMed]
- Miura H, Nakajima N, Takahashi H, et al. Therapeutic strategy for secondary lung cancer. Kyobu Geka 2010;63:956-61. [PubMed]
- Kurasawa T. The coexistence of pulmonary tuberculosis and lung cancer. Nihon Rinsho 1998;56:3167-70. [PubMed]
- Smego RA Jr, Foglia G. Actinomycosis. Clin Infect Dis 1998;26:1255-61. [Crossref] [PubMed]
- Matthews MJ, Kanhouwa S, Pickren J, et al. Frequency of residual and metastatic tumor in patients undergoing curative surgical resection for lung cancer. Cancer Chemother Rep 3 1973;4:63-7. [PubMed]
- Lutwyche VU. The differential diagnosis of carcinoma of the bronchus and pulmonary tuberculosis. Lancet 1954;266:693-5. [Crossref] [PubMed]
- Bakheet SM, Saleem M, Powe J, et al. F-18 fluorodeoxyglucose chest uptake in lung inflammation and infection. Clin Nucl Med 2000;25:273-8. [Crossref] [PubMed]
- Huang SF, Li CP, Feng JY, et al. Increased risk of tuberculosis after gastrectomy and chemotherapy in gastric cancer: a 7-year cohort study. Gastric Cancer 2011;14:257-65. [Crossref] [PubMed]
- Zavala DC. Diagnostic fiberoptic bronchoscopy: Techniques and results of biopsy in 600 patients. Chest 1975;68:12-9. [Crossref] [PubMed]
- Conde MB, Soares SL, Mello FC, et al. Comparison of sputum induction with fiberoptic bronchoscopy in the diagnosis of tuberculosis: experience at an acquired immune deficiency syndrome reference center in Rio de Janeiro, Brazil. Am J Respir Crit Care Med 2000;162:2238-40. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer 2004;101:2503-8. [Crossref] [PubMed]
- Mori T, Misago N, Yamamoto O, et al. Expression of nestin in dermatofibrosarcoma protuberans in comparison to dermatofibroma. J Dermatol 2008;35:419-25. [Crossref] [PubMed]
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol 2012;148:1055-63. [Crossref] [PubMed]
- Malhotra B, Schuetze SM. Dermatofibrosarcoma protruberans treatment with platelet-derived growth factor receptor inhibitor: a review of clinical trial results. Curr Opin Oncol 2012;24:419-24. [Crossref] [PubMed]