Receptor-binding domain as a target for developing SARS vaccines
Introduction
Severe acute respiratory syndrome (SARS) caused by a novel human coronavirus (SARS-CoV) emerged from Guangdong Province, China, in late 2002. By the end of 2003, it had spread to more than 30 countries, affecting 8,096 people and causing 774 deaths (a case fatality rate of about 10%) (1-3). Although the global SARS pandemic was brought under control in July 2003, reports of sporadic cases in China from late 2003 to early 2004 (4) raised concerns about the reemergence of SARS-CoV through either zoonotic reintroduction or laboratory escape (5,6).
Most recently, a close relative of SARS-CoV, Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV), has been identified as the pathogen causing outbreaks of SARS-like illness with a case fatality rate of 55% in the Middle East, Europe and Africa (7-9). These reports have raised concerns over the possibility of a reemergence of SARS-CoV and, hence, call for the development of effective and safe SARS vaccines to combat any future SARS pandemic (10).
Identification of the receptor-binding domain (RBD) in the SARS-CoV spike protein and its role in viral entry into the target cell
SARS-CoV is a single, nonsegment and positive-stranded RNA virus with envelope. Its genomic RNA consists of 29,736 nucleotides, two thirds of its 5'-encoding nonstructural RNA replicase polyprotein and one third of its 3'-encoding structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins (11).
The S protein of SARS-CoV is a type I transmembrane envelope glycoprotein (Env), which plays a significant role in receptor binding, membrane fusion and virus entry. The entry of SARS-CoV is initiated by binding of the S protein to the cellular receptor angiotensin-converting enzyme 2 (ACE2) (12). The virion-ACE2 complex is then translocated to endosomes. Cathepsin L inhibitors could significantly block the entry of SARS-CoV, indicating that S protein is cleaved by endosomal acid proteases (cathepsin L) to activate its fusion activity (13). After the fusion peptide (FP) inserts into the endosomal membrane, the heptad repeat 1 and 2 (HR1 and HR2) domains in the S protein interact with each other to form a six-helix bundle (6-HB) core, which brings the viral envelope and the cellular plasma membrane into close proximity for fusion. The viral RNA genome is then released into the cytoplasm (14,15) (Figure 1). Alternatively, SARS-CoV may also enter the target cell through plasma membrane fusion in a manner similar to HIV. After the S1 subunit of SARS-CoV S protein binds to ACE2, the S2 subunit changes conformation by inserting the fusion peptide into the plasma membrane. The HR2 domain interacts with the HR1 trimer to form 6-HB core, leading to the fusion between the viral envelope and the cellular plasma membrane (16).
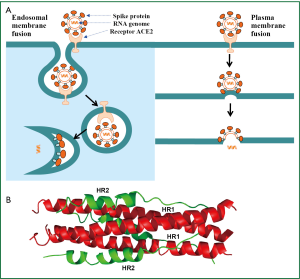
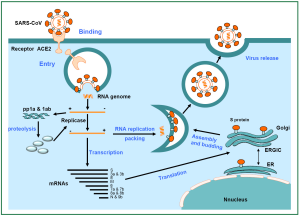
Subsequently, the genomic RNA genome serves as a template for synthesizing full-length and subgenomic-length negative-strand RNAs, which then serve as template for synthesis of mRNA. Viral proteins are translated and then transported to the lumen of the ER-Golgi intermediate compartment (ERGIC) (18). From genomic RNA and N protein in the cytoplasm, viral nucleocapsids are assembled. Through exocytosis, virions are then released from the cell (Figure 2).
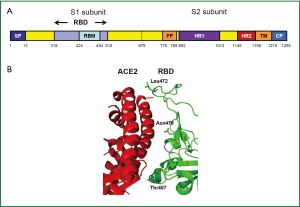
The SARS-CoV S protein consists of S1 surface subunit, which is responsible for receptor-binding, and S2 transmembrane subunit, which mediates membrane fusion. A fragment spanning the residues 318-510 in S1 subunit is the minimal RBD (19,20). The RBD contains a loop region (residues 424-494), termed receptor-binding motif (RBM) (Figure 3A), which makes complete contact with the receptor ACE2. Interestingly, the RBM region is tyrosine-rich. Six out of 14 residues of RBM that are in direct contact with ACE2 are tyrosines. Two residues in RBM, Asn479 and Thr487, determine SARS disease progression and SARS-CoV tropism (22,23). Substitutions of these two residues may change the animal-to-human or human-to-human transmissibility of the virus (Figure 3B) (21). The multiple cysteine residues in the RBD region are important for maintaining the functional conformation of the RBDs of SARS-CoV (21) and MERS-CoV (24).
RBD contains the critical neutralizing domain (CND) that induces potent and broad neutralizing antibodies
Our previous studies have demonstrated that the antisera isolated from SARS patients and from animals immunized with inactivated SARS-CoV vaccine could react significantly with the RBD of the SARS-CoV S protein, indicating that the RBD possesses potent neutralizing activity (25). Depletion of RBD-specific antibodies from patient or rabbit immune sera by immunoadsorption resulted in significant reduction of the serum-mediated neutralizing activity (26). Antibodies purified from the antisera against SARS-CoV significantly inhibited RBD binding to ACE2, and the affinity-purified anti-RBD antibodies exhibited a relatively higher potency in neutralizing infectivity (26,27). All these results suggest that the RBD of S protein contains a CND and that RBD may therefore be used as an immunogen to induce neutralizing antibodies against SARS-CoV.
We previously found that a single amino acid substitution in the RBD, such as R441A, was able to abolish the immunogenicity of RBD to induce neutralizing antibodies in immunized mice and rabbits and that RBD bearing R441A mutation could not bind to the soluble and cell-associated ACE2, suggesting that some critical residues in the RBM of the RBD are also important residues in the CND. However, as demonstrated by Ye et al., the mutation of R453A in RBM abolished viral entry, but retained the capacity for inducing neutralizing antibodies, suggesting that some residues in CND may not participate in the RBD-receptor interaction (28).
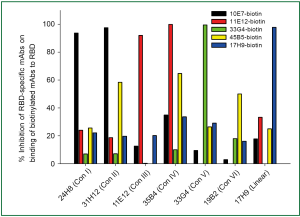
A panel of 27 RBD-specific monoclonal antibodies (mAbs) was isolated from mice immunized with RBD conjugated with IgG Fc (RBD-Fc). Among these, mAbs 4D5 and 17H9 could recognize linear epitopes of RBD, but they showed no neutralizing activity. Using a binding competition assay, the remaining 25 RBD-specific mAbs could be divided into six distinct groups based on the conformation of the epitopes in RBD that they recognized (i.e., Conf I-VI) (Figure 4). We found that only the mAbs recognizing Conf IV and V could efficiently block RBD binding to ACE2, suggesting that the residues in their epitopes are also involved in RBD-ACE2 interaction. The mAbs that recognized Conf I and II did not significantly affect RBD binding with ACE2. Still, they possessed potent neutralizing activities, indicating that these mAbs could inhibit SARS-CoV infection without interfering in RBD-ACE2 interaction (27). These findings suggest that the RBD of SARS-CoV S protein contains multiple conformational epitopes responsible for eliciting potent neutralizing responses and can therefore serve as a target for development of SARS vaccines.
RBD-based SARS vaccines
Although the inactivated virus-based, DNA-based and viral vector-based vaccine candidates could induce effective neutralizing antibody responses, their safety is a major concern for further development. A double-inactivated SARS-CoV vaccine was proven to elicit eosinophilic and immunoenhancing pathology (29). The SARS-CoV nucleocapsid protein (NP) in the inactivated vaccine may be responsible for this unwanted property (30,31). The full-length S protein may also not be safe. For example, the vaccine candidates containing recombinant S protein are able to cause Th2-mediated immunopathology (32) or some immune enhancement in vaccinated animals (33).
Based on our previous studies, we believe that a RBD-based SARS vaccine is the most effective and safest. First, we have demonstrated that the recombinant RBD expressed in mammalian cells linked to human IgG Fc (RBD-Fc) could induce highly potent neutralizing antibodies in vaccinated mice and rabbits (34). Second, the recombinant RBD without Fc tag expressed in mammalian (293T) cells, insect (Sf9) cells, and E. coli, respectively, could induce highly potent neutralizing antibody responses and complete protective immunity against SARS-CoV challenge in mice. Third, a 219-mer (residues 318-536) RBD protein expressed in Chinese hamster ovary (CHO)-K1 cells (RBD219-CHO) and a 193-mer (residues 318-510) RBD stably expressed in CHO cells (RBD193-CHO) could induce strong humoral and cellular immune responses and protection in all vaccinated mice (35,36). Fourth, a recombinant adeno-associated virus (rAAV)-based RBD (RBD-rAAV) vaccine could induce humoral immune response with neutralizing activity in intramuscular (i.m.)-vaccinated BALB/c mice (37). The intranasal (i.n.) application of RBD-rAAV vaccine could induce more potent SARS-CoV-specific systemic and mucosal immune responses than i.m. administration (38). Fifth, priming with RBD-rAAV vaccine and boosting with RBD-specific peptides for T cell epitopes significantly elevated anti-SARS-CoV humoral and cellular immune responses (39). Sixth, RBD-based SARS vaccine could induce high titer of S-specific antibodies with long-term neutralizing activity and long-term protective immunity in an animal model (40). All these results indicate that RBD-based vaccines have good potential to be further developed as an effective and safe vaccine for preventing SARS-CoV infection and combating the recurrence of SARS pandemic in the future.
Conclusions and prospect
Considering the recent outbreaks of SARS-like disease caused by the newly emerged MERS-CoV and the potential of future recurrence of SARS, development of effective and safe vaccines against SARS-CoV remains a high priority. Our previous studies have demonstrated that the RBD in the S1 subunit of the SARS-CoV S protein contains the CND that can induce highly potent humoral and cellular immune responses, particularly cross-neutralizing antibodies and strong protective immunity. Therefore, RBD-based vaccines show considerable promise for further development as a highly effective SARS vaccine. Furthermore, this strategy could also be employed for the development of vaccines against other emerging infectious diseases caused by enveloped viruses with class I membrane fusion proteins, such as avian influenza A(H7N9) virus (41-43) and MERS-CoV (7,24).
Recently, Chan et al. (44) have demonstrated that sera collected from convalescent SARS patients may contain cross-reactive antibodies against MERS-CoV detected by both immunofluorescent and neutralizing antibody tests. Based on bioinformatics analysis, they anticipated that the B-cell epitope that elicited cross-reactive antibodies may be located in the S2 subunit HR2 domain of MERS-CoV. Most recently, we have shown that the mAbs specific for the RBD of SARS-CoV S protein exhibited no cross-reactive or cross-neutralizing activity against MERS-CoV, suggesting that the RBDs of SARS-CoV and MERS-CoV S proteins may not contain the epitopes for inducing cross-reactive antibody responses (45). Therefore, the design and development of a RBD-based vaccine against MERS-CoV will need to follow an experimental path similar to that of our RBD-based SARS vaccine.
Acknowledgements
This study was supported by grants from the 973 Programme of China (#2012CB519001), the Chinese Ministry of Science & Technology, Hong Kong, Macau, and Taiwan Collaborative Programs (201200007673), and NIH of the United States (R01 AI098775).
Disclosure: The authors declare no conflict of interest.
References
- Zhong NS, Zheng BJ, Li YM, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 2003;362:1353-8. [PubMed]
- Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 2003;361:1319-25. [PubMed]
- Butler D. SARS veterans tackle coronavirus. Nature 2012;490:20. [PubMed]
- Liang G, Chen Q, Xu J, et al. Laboratory diagnosis of four recent sporadic cases of community-acquired SARS, Guangdong Province, China. Emerg Infect Dis 2004;10:1774-81. [PubMed]
- Du L, He Y, Zhou Y, et al. The spike protein of SARS-CoV--a target for vaccine and therapeutic development. Nat Rev Microbiol 2009;7:226-36. [PubMed]
- Jiang S, Bottazzi ME, Du L, et al. Roadmap to developing a recombinant coronavirus S protein receptor-binding domain vaccine for severe acute respiratory syndrome. Expert Rev Vaccines 2012;11:1405-13. [PubMed]
- Zaki AM, van Boheemen S, Bestebroer TM, et al. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012;367:1814-20. [PubMed]
- Gallagher T, Perlman S. Public health: Broad reception for coronavirus. Nature 2013;495:176-7. [PubMed]
- WHO. Novel coronavirus infection - update (Middle East respiratory syndrome- coronavirus). Available online: http://www.who.int/csr/don/2013_05_29_ncov/en/index.html. accessed May 30, 2013.
- Jiang S, Lu L, Du L. Development of SARS vaccines and therapeutics is still needed Future Virol 2013;8:1-2.
- Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev 2005;69:635-64. [PubMed]
- Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450-4. [PubMed]
- Simmons G, Gosalia DN, Rennekamp AJ, et al. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A 2005;102:11876-81. [PubMed]
- Yang ZY, Huang Y, Ganesh L, et al. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J Virol 2004;78:5642-50. [PubMed]
- Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res 2008;18:290-301. [PubMed]
- Liu S, Xiao G, Chen Y, et al. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 2004;363:938-47. [PubMed]
- Xu Y, Lou Z, Liu Y, et al. Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J Biol Chem 2004;279:49414-9. [PubMed]
- Stertz S, Reichelt M, Spiegel M, et al. The intracellular sites of early replication and budding of SARS-coronavirus. Virology 2007;361:304-15. [PubMed]
- Xiao X, Chakraborti S, Dimitrov AS, et al. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem Biophys Res Commun 2003;312:1159-64. [PubMed]
- Wong SK, Li W, Moore MJ, et al. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem 2004;279:3197-201. [PubMed]
- Li F, Li W, Farzan M, et al. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005;309:1864-8. [PubMed]
- Qu XX, Hao P, Song XJ, et al. Identification of two critical amino acid residues of the severe acute respiratory syndrome coronavirus spike protein for its variation in zoonotic tropism transition via a double substitution strategy. J Biol Chem 2005;280:29588-95. [PubMed]
- Li W, Zhang C, Sui J, et al. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J 2005;24:1634-43. [PubMed]
- Jiang S, Lu L, Du L, et al. A predicted receptor-binding and critical neutralizing domain in S protein of the novel human coronavirus HCoV-EMC. J Infect 2013;66:464-6. [PubMed]
- He Y, Zhou Y, Siddiqui P, et al. Inactivated SARS-CoV vaccine elicits high titers of spike protein-specific antibodies that block receptor binding and virus entry. Biochem Biophys Res Commun 2004;325:445-52. [PubMed]
- He Y, Zhu Q, Liu S, et al. Identification of a critical neutralization determinant of severe acute respiratory syndrome (SARS)-associated coronavirus: importance for designing SARS vaccines. Virology 2005;334:74-82. [PubMed]
- He Y, Lu H, Siddiqui P, et al. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J Immunol 2005;174:4908-15. [PubMed]
- Yi CE, Ba L, Zhang L, et al. Single amino acid substitutions in the severe acute respiratory syndrome coronavirus spike glycoprotein determine viral entry and immunogenicity of a major neutralizing domain. J Virol 2005;79:11638-46. [PubMed]
- Bolles M, Deming D, Long K, et al. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol 2011;85:12201-15. [PubMed]
- Deming D, Sheahan T, Heise M, et al. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med 2006;3:e525. [PubMed]
- Yasui F, Kai C, Kitabatake M. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol 2008;181:6337-48. [PubMed]
- Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One 2012;7:e35421. [PubMed]
- Jaume M, Yip MS, Kam YW, et al. SARS CoV subunit vaccine: antibody-mediated neutralisation and enhancement. Hong Kong Med J 2012;18 Suppl 2:31-6. [PubMed]
- He Y, Zhou Y, Liu S, et al. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun 2004;324:773-81. [PubMed]
- Du L, Zhao G, Li L, et al. Antigenicity and immunogenicity of SARS-CoV S protein receptor-binding domain stably expressed in CHO cells. Biochem Biophys Res Commun 2009;384:486-90. [PubMed]
- Du L, Zhao G, Chan CC, et al. A 219-mer CHO-expressing receptor-binding domain of SARS-CoV S protein induces potent immune responses and protective immunity. Viral Immunol 2010;23:211-9. [PubMed]
- Du L, He Y, Wang Y, et al. Recombinant adeno-associated virus expressing the receptor-binding domain of severe acute respiratory syndrome coronavirus S protein elicits neutralizing antibodies: Implication for developing SARS vaccines. Virology 2006;353:6-16. [PubMed]
- Du L, Zhao G, Lin Y, et al. Intranasal vaccination of recombinant adeno-associated virus encoding receptor-binding domain of severe acute respiratory syndrome coronavirus (SARS-CoV) spike protein induces strong mucosal immune responses and provides long-term protection against SARS-CoV infection. J Immunol 2008;180:948-56. [PubMed]
- Du L, Zhao G, Lin Y, et al. Priming with rAAV encoding RBD of SARS-CoV S protein and boosting with RBD-specific peptides for T cell epitopes elevated humoral and cellular immune responses against SARS-CoV infection. Vaccine 2008;26:1644-51. [PubMed]
- Du L, Zhao G, He Y, et al. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine 2007;25:2832-8. [PubMed]
- Gao R, Cao B, Hu Y, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 2013;368:1888-97. [PubMed]
- Gao HN, Lu HZ, Cao B, et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 2013;368:2277-85. [PubMed]
- Liu Q, Lu L, Sun Z, et al. Genomic signature and protein sequence analysis of a novel influenza A (H7N9) virus that causes an outbreak in humans in China. Microbes Infect 2013;15:432-9. [PubMed]
- Chan KH, Chan JF, Tse H, et al. Cross-reactive antibodies in convalescent SARS patients’ sera against the emerging novel human coronavirus EMC (2012) by both immunofluorescent and neutralizing antibody tests. J Infect 2013;67:130-40. [PubMed]
- Du L, Ma C, Jiang S. Antibodies induced by receptor-binding domain in spike protein of SARS-CoV do not cross-neutralize the novel human coronavirus hCoV-EMC. J Infect 2013. [Epub ahead of print]. [PubMed]


