Pulmonary cryptococcosis manifesting as diffuse air-space consolidations in an immunocompetent patient
Introduction
Cryptococcus is one of the most common infections in immunocompromised patients, such as those with AIDS, organ transplantation recipients, and patients with hematologic malignancies. However, it can also occur in immunocompetent patients, which accounts for up to 35% of pulmonary cryptococcosis (1). Regardless of the host’s immune status, the most common radiologic features of pulmonary cryptococcosis are variable-sized nodules or masses, followed by consolidation. Those immunocompromised patients tend to have a more diffuse disease, demonstrated by multiple consolidations (2,3). However, in immunocompetent patients, pulmonary cryptococcosis commonly manifests localized air-space consolidation (4). Therefore, we present a rare case of pulmonary cryptococcosis that displayed diffuse air-space consolidations in an immunocompetent patient.
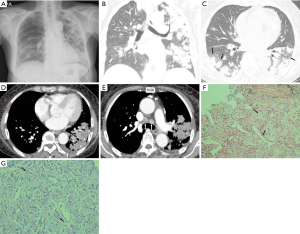
Case presentation
A 73-year-old woman presenting with a two-month history of cough was admitted to our institute. There were no other symptoms such as hemoptysis, fever, or weight loss. Her medical history included pancreatic cancer diagnosed 7 years prior. After a Whipple operation and adjuvant chemotherapy, there was no evidence of disease for the past 7 years. She had not been medicated with any steroids or anti-diabetics, and was a non-smoker. On laboratory data, WBC counts were within normal range (7,890/µL) and neutrophil dominant (80%). Percentage of lymphocytes was 11%. C-reactive protein level increased as 100.15 mg/L. Liver function tests including prothrombin time, AST and ALT were within normal range. Serum BUN and creatinine were also normal. Antibody titers against HIV and other viruses were also negative. On initial chest radiography taken at admission, patchy increased opacities and diffuse patchy consolidations were noted in both lungs (Figure 1A). Subsequent contrast-enhanced chest computed tomography (CT) scans showed multifocal, segmental, or subsegmental consolidations and multiple ill-defined, subcentimeter, randomly distributed small nodules in both lungs, which were predominant and extensive in the left lung (Figure 1B). Some of the small nodules and consolidations were conglomerated and accompanied by surrounding ground-glass opacities (CT halo sign) (Figure 1C), of which some were combined with mucus-filled bronchial trees (CT mucous bronchogram sign) (Figure 1D). A small left pleural effusion and several enlarged bilateral mediastinal and left hilar lymph nodes were also revealed (Figure 1E). Based on her chronic symptoms and unresolving radiologic findings, we first suggested the possibility of a pulmonary malignancy such as invasive adenocarcinoma and lymphoma. When considering her past medical history, intra-thoracic metastasis from pancreatic cancer was also possible. Fungal infection, atypical pneumonia or diffuse alveolar pulmonary hemorrhage was also included in the differential diagnosis. Abdominal enhanced CT showed no evidence of tumor recurrence. Serum tumor markers were also within normal range. The microbiological examination of sputum revealed negative AFB stain, and few nonpathogenic Neisseria and non-pneumococcal alpha-hemolytic Streptococci on culture. Before tissue confirmation, she had been treated with empirical antibiotics for 6 days, starting from the first day of admission. However, her cough had not subsided and she subsequently developed a fever up to 38 °C. There were no changes in the patchy opacities or diffuse patchy consolidations on follow-up chest radiography. The diagnostic bronchoscopy performed on hospital day 5 showed no endobronchial lesions. On hospital day 7, a CT-guided transthoracic fine needle biopsy was performed at the consolidation of the left lower lobe. There were no post-procedural complications such as pneumothorax or hemothorax. A histopathologic examination of the biopsy specimen revealed numerous, encapsulated yeast-like fungi on both periodic acid-Schiff (PAS) and Gomori Methenamin silver stains, which were consistent with Cryptococcus (Figure 1F,G). Lumbar puncture or brain imaging was not performed due to negative serum cryptococcal antigen test and negative neurological symptoms or signs. At first, an antifungal agent, amphotericin B, was administrated intravenously. Two days later, the fever had subsided. Her cough and chest radiographic findings gradually improved during follow-up. She continued to receive an intravenous infusion of amphotericin B, and then was eventually switched to an oral medication, fluconazole. On hospital day 23, she was discharged and continued to take the oral fluconazole during 6 months for maintenance. There was no evidence of relapse over the 12-month follow-up period.
Discussion
In pulmonary cryptococcosis, there are three patterns of radiologic findings. The first is a nodule or mass, the second is consolidation and the third is reticular opacities. Of these findings, the most common feature is variable-sized nodules or masses in both immunocompetent and immunocompromised patients. The proportion of pulmonary nodules and consolidation was not significantly different between the two groups. Associated radiologic findings in pulmonary cryptococcosis include cavitation, ground-glass opacities, CT halo sign, mediastinal lymphadenopathy, and pleural effusion. The cavitation significantly occurs in immunocompromised patients. In our case, extensive consolidations and several small nodules were observed in both lungs. There were no cavitations within nodules or consolidation, which was consistent with previous immunocompetent cases. These large consolidations seem to be a CT finding common to the immunocompromised. In pulmonary cryptococcosis, when there is a pathologic granulomatous reaction, mediastinal lymphadenopathy and an ill-defined mass, segmental consolidation, or an infiltrative mass is common (5). Considering the extensive consolidation and mediastinal lymphadenopathy seen in our case, it is likely that there was a significant granulomatous reaction in our patient.
In a CT study of pulmonary cryptococcosis in immunocompetent patients, pulmonary nodules have been reported to be multiple, smaller than 10 mm in diameter, and have a well-defined, smooth margin. Nodules commonly involved less than 10% of the lung parenchyma, and were distributed in the middle and upper lungs, bilaterally (6). Another study reported that nodules have a well-defined smooth margin, whereas the lesion size can range from 5 to 52 mm in diameter. It was also reported that CT findings were influenced by patient age. Patients younger than 44 years had multiple pulmonary nodules, consolidation, or cavitation. However, patients older than 44 years had only one or two peripheral nodules (7). Although they gave no reason for their suggestion, we think that this discrepancy may be explained by a granulomatous reaction, which may be more active at a younger age. However, in our case, a 73-year-old patient showed CT findings of massive consolidations and multiple bilateral small nodules. Although she was at an advanced age, these findings were likely due to an active granulomatous reaction.
When air-space consolidations are seen in patients with no symptoms or signs of pulmonary infection, the radiologist should exclude the possibility of malignancy, such as invasive mucinous adenocarcinoma, which has CT findings including consolidations and multifocal or multi-lobular nodules or masses. Yet, there are some features that favor malignancy over pneumonia. The first is a coexisting nodule and a peripheral distribution of consolidation (8). In our case, combined several subcentimeter nodules with massive consolidations were initially suggestive of malignancy. However, there are some limitations to our case, because the previous comparison between pneumonia and malignancy was primarily community-acquired or aspiration pneumonia. The second finding favoring malignancy is stretching, squeezing, sweeping, and widening of the branching angle of an air-filled bronchus within an area of consolidation, which was not detectable in our case (9). Instead, our case showed mucus filling bronchial trees within the consolidation on CT, a so-called CT mucous bronchogram sign. This refers to low attenuation tree-like branching structures within consolidations or collapsed lungs. It is mostly indicative of bronchogenic carcinoma or an impaired mucociliary transport condition. However, there has been a report of pulmonary cryptococcosis showing a CT mucous bronchogram sign due to Cryptococcus manifesting as a large central mass with complete obstruction of the right middle bronchus (10). Finally, the CT halo sign, which has been considered a characteristic finding of invasive aspergillosis in the past, was also visible in our case. Unfortunately, the CT halo sign itself does not appear to differentiate malignant and benign conditions. In pulmonary cryptococcosis, it results from disease infiltration into the surrounding lung and is visible in both immunocompromised and immunocompetent groups, whereas the CT halo sign of invasive mucinous adenocarcinoma is due to the tumor’s lepidic growth pattern (11).
In conclusion, we reported a case of pulmonary cryptococcosis displaying diffuse air-space consolidations with CT halo and mucous bronchogram signs in an immunocompetent patient. Although diffuse air-space consolidations are usually seen in immunocompromised patients, the possibility of pulmonary cryptococcosis should be considered when a patient with a normal immune status presents without respiratory symptoms and/or when CT halo and mucous bronchogram signs are accompanied by consolidation on imaging.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Aberg JA, Mundy LM, Powderly WG. Pulmonary cryptococcosis in patients without HIV infection. Chest 1999;115:734-40. [Crossref] [PubMed]
- Chang WC, Tzao C, Hsu HH, et al. Pulmonary cryptococcosis: comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest 2006;129:333-40. [Crossref] [PubMed]
- Khoury MB, Godwin JD, Ravin CE, et al. Thoracic cryptococcosis: immunologic competence and radiologic appearance. AJR Am J Roentgenol 1984;142:893-6. [Crossref] [PubMed]
- Lee CY, Kim H, Kim JS, et al. HRCT finding of pulmonary cryptococcosis in immune competent patients. J Korean Radiol Soc 2001;44:167-71. [Crossref]
- Su CT, Chen LK, Tsai YF, et al. Disseminated cryptococcosis with pulmonary and marrow involvement mimicking radiological features of malignancy. J Chin Med Assoc 2004;67:89-92. [PubMed]
- Lindell RM, Hartman TE, Nadrous HF, et al. Pulmonary cryptococcosis: CT findings in immunocompetent patients. Radiology 2005;236:326-31. [Crossref] [PubMed]
- Fox DL, Müller NL. Pulmonary cryptococcosis in immunocompetent patients: CT findings in 12 patients. AJR Am J Roentgenol 2005;185:622-6. [Crossref] [PubMed]
- Aquino SL, Chiles C, Halford P. Distinction of consolidative bronchioloalveolar carcinoma from pneumonia: do CT criteria work? AJR Am J Roentgenol 1998;171:359-63. [Crossref] [PubMed]
- Jung JI, Kim H, Park SH, et al. CT differentiation of pneumonic-type bronchioloalveolar cell carcinoma and infectious pneumonia. Br J Radiol 2001;74:490-4. [Crossref] [PubMed]
- Suwatanapongched T, Sangsatra W, Boonsarngsuk V, et al. Clinical and radiologic manifestations of pulmonary cryptococcosis in immunocompetent patients and their outcomes after treatment. Diagn Interv Radiol 2013;19:438-46. [PubMed]
- Lee YR, Choi YW, Lee KJ, et al. CT halo sign: the spectrum of pulmonary diseases. Br J Radiol 2005;78:862-5. [Crossref] [PubMed]


