Role of molecular imaging with positron emission tomographic in aortic aneurysms
Introduction
Although the past few decades have witnessed great progress in clinical aortic imaging, medical care and surgical techniques, patients with aortic aneurysms (AA) continue to have high rates of morbidity and mortality. Based on current AA consensus guidelines, aortic size is the guiding metric for use in diagnosis and surveillance, and criterion for elective surgical intervention (1). The standard anatomic-based imaging approaches, which mainly focus on measures of aortic size, reflect relatively late stage pathology, when structural dilation has already occurred. Beyond anatomic imaging, focusing on primary and aggravating pathological functional processes such as aortic wall inflammatory activity and perturbed biomechanics requires application of molecular and functional imaging techniques, such as hybrid positron emission tomographic (PET) imaging. This approach has the potential to accelerate diagnosis, refine prognosis, and guide treatments. The ability to identify aortic characteristics or underlying processes associated with increased aortic growth would be tremendously valuable, both in guiding selection of patients for prophylactic surgical repair and for optimizing timing of intervention to prevent aortic complications.
Thoracic AA
Clinical problem
Thoracic aortic aneurysms (TAAs) are silent, but life-threatening conditions (2). Approximately 95% of TAAs are asymptomatic before the occurrence of often fatal acute complications, such as aortic dissection or rupture (3-5). Although prophylactic surgical correction is guided by anatomic measures of aortic size, diameter alone poorly predicts the risk for life-threatening complications (6,7). For example, it has been recently demonstrated that up to 60% of genetically-mediated TAA patients who developed aortic dissections had aortic diameters below the conventional threshold of 5.0 cm for prophylactic surgery (8,9). In non-genetic TAAs, is has been demonstrated that nearly 60% of type A dissection patients had aortic diameters <5.5 cm, the current cutoff for prophylactic surgery in this population; and 40% had diameters <5.0 cm. Moreover, recent data from our group and others have indicated that risk for aortic complications remains elevated even after prophylactic AA surgery. For example, among a cohort of 1,991 patients with genetically-mediated TAAs [22% Marfan Syndrome (MFS), 39% bicuspid aortic valve (BAV)], 52% of dissections, including 68% in MFS, occurred in patients who had previously undergone prophylactic graft repair of other aortic segments (8). Taken together, given the poor predictive value of aortic size to risk stratify patients, additional markers of thoracic aortic vulnerability are crucial to better encompass patients’ risk for TAA complications.
Scope of the problem
According to the Centers for Disease Control and Prevention, AAs account for 43,000 to 47,000 deaths annually (9). In patients with TAAs, nearly 40% die in the field due to aortic rupture or dissection, and out-of-hospital mortality is ≥1–2% per hour (10). Among those that reach a hospital alive, the perioperative mortality for emergent surgical repair is ~25% (11,12). Each year, an estimated 10–11 per 100,000 people are diagnosed with a TAA (13). This number is expected to rise due to recently adopted imaging guidelines employing chest CT for lung cancer screening, which will incidentally detect more TAAs, previously undiagnosed until a lethal aortic complication. An estimated 7 million patients meet criteria for chest CT screening, which will detect an estimated 280,000 new TAAs (14). For all these reasons, the National Institutes of Health (NIH) has classified this disease as a significant burden on the health care system. In light of these data, improved stratification of patients at risk for TAAs and optimization of management are essential, and represent an important public health and research focus. Current risk stratification is reliant on routine noninvasive imaging assessment of aortic anatomy—aortic size. However, it is evident that standard anatomic-based imaging assessment of aortic size alone does not capture underlying functional processes related to aneurysm dilation, such as inflammation.
Basis for arterial fluorine-2-deoxy-D-glucose (FDG) uptake for localization of inflammation
Accelerated glucose metabolism is the basis for the use of FDG with PET in detecting inflammation. FDG is taken up by glucose-requiring activated inflammatory cells via the glucose transporter protein system (GLUT). After FDG enters the inflammatory cell, it undergoes phosphorylation by the hexokinase enzyme system to FDG-6-phosphate. However, unlike glucose-6-phosphate, FDG-6-phosphate is not metabolized further along the glycolytic pathway (i.e., not dephosphorylated by glucose-6-phosphatase), resulting in metabolic trapping of 18 FDG-6-PO4 in the cell in direct proportion to its overall metabolic state (15).
Activated macrophages and other inflammatory cells demand substantially greater glucose for accelerated cellular metabolic processes. Importantly, inflammatory cells have a distinctly higher rate of glucose uptake than non-inflammatory cells residing within an arterial wall, resulting in increased FDG uptake and facilitating the detection of an inflamed locus. In addition, the hypoxic plaque environment favors anaerobic metabolism, and in turn, stimulates macrophages to metabolize glucose rather than oxygen-dependent metabolism of free fatty acids (16). Thus, glucose becomes the main energy source for inflammatory cells within an inflamed arterial wall (17).
Several studies have established a strong correlation between arterial FDG uptake and macrophage content in a variety of animal models (18-20). In murine experiments, a strong correlation between FDG uptake and gene expression of inflammatory molecular markers has been demonstrated (21). Building on these early findings, in rabbits, it has been shown that inflamed aortic lesions accumulate up to 20 times more FDG than non-inflamed arterial lesions, thereby enabling reliable noninvasive detection of inflamed loci within arterial wall with FDG-PET (20). From these studies, it becomes clear that the PET derived FDG signal can be reliably used as a beacon for arterial wall inflammation.
Clinical studies of FDG-PET imaging of inflammation in thoracic AA
Though molecular inflammation imaging has been mostly been performed in the context of abdominal aortic aneurysm (AAA), a few studies have demonstrated utility of inflammation imaging in TAAs (see Table 1). For example, one clinical study demonstrated a correlation of FDG-avidity in the aortic wall and progression of AA. Blockmans et al. studied 46 patients with biopsy proven giant cell arteritis and FDG PET scan at baseline. After a mean follow up of 46.7±29.9 months, a single CT scan of the aorta was performed. They noted patients with increased FDG uptake in the aorta at diagnosis of giant cell arteritis had a significantly larger thoracic aortic diameter (in both ascending and descending aorta) and volume compared those with minimal to no FDG uptake (25). In multivariate regression model, FDG uptake was significantly associated with late volume of the thoracic aorta.
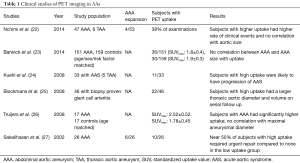
Full table
In another study, by Kuehl et al., 33 patients with acute aortic syndrome (thoracic aortic aneurysm 5, thoracic aortic dissection 14, penetrating aortic ulcer 8, intramural hematoma 6) and FDG-PET scan at baseline were studied. Of those with increased aortic uptake [standardized uptake value (SUV)max >2.5], 9 of 11 patients (82%) had progression of disease or received definitive surgical treatment, while a majority of patients (55%) without elevated tracer uptake displayed stability or regression of disease and did not require intervention at follow-up (24).
AAA
Clinical problem and magnitude
AAA is a localized dilation of the aorta with a diameter of 3 cm or greater. Advanced AAA eventually leads to rupture, which is associated with a mortality of 90% (28). AAA leads to 30,000 deaths every year, of those 15,000 are due to rupture (29,30). The mechanism of AAA involves changes in the aortic wall’s extracellular matrix thought to be associated with increased matrix metalloproteinases (MMPs). Inflammation is associated with an increase in these enzymes that are produced by macrophages, lymphocytes, and mast cells (31,32). Prevalence of AAA is higher in men and with advanced age (33). Other risk factors for AAA include white race, obesity, family history, smoking, and known atherosclerosis. The US Preventive Task Force recommends screening for AAA in men aged
Clinical studies of FDG-PET imaging of inflammation in abdominal AA
There have been more studies in AAA utilizing PET imaging given the established association of the presence of inflammation with AAAs (see Table 1). FDG is the most commonly used agents and is recognized as a surrogate for aortic wall inflammation. Sakalihasan et al. first reported the correlation of FDG-PET to clinical aortic events. In 26 patients with AAA, 10 patients demonstrated increased uptake of FDG. Those with no or minimal uptake did not require urgent surgery, whereas, 5 out of the 10 patients who showed increased uptake required urgent surgery within 2 to 30 days (27).
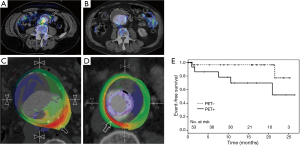
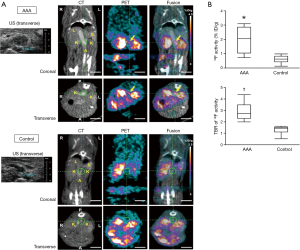
In another well-known study, Nchimi et al. studied 53 patients with descending AAA =131 areas, TAA =54 areas. This study showed that nearly one third of subjects had increased (i.e., positive) FDG PET uptake. After 11 months of follow-up to monitor for clinical events (defined as rupture, dissection, or aortic growth >1 cm), those with increased FDG uptake had a significant increase in clinical events compared to those without uptake [5 of 18 (28%) versus 2 of 35 (6%); P=0.03; see Figure 1]. In addition, finite element analyses were performed and showed that areas of high FDG uptake did not significantly correlate with areas that harbored the highest wall stress (including stress/strength index) (22). This latter point suggests that inflammation (i.e., FDG uptake) may provide additional information to biomechanical wall stress and lumen diameter.
Truijers et al. conducted a retrospective study of 17 asymptomatic patients with AAA (maximal diameter >3.0 cm) and an age-matched control group that had FDG-PET performed for staging of primary lung cancer. They compared the SUVmax between these two groups. Those with AAA had a significantly higher SUV-max compared to the control group (26). These findings were reproducible and reported by other groups as well (36,37). On other hand, Palombo et al. evaluated the PET-measured SUV in the aortic wall of 50 male patients with asymptomatic AAA and 44 age-matched controls (24 males, 20 females), 44 age-matched controls subjects (mean age: 71 years, range: 59-85 years, 24 males, 20 females) with no known atherosclerotic disease. They noted a lower mean and SUVmax in those with AAA compared to adjacent non-aneurysmal segments in controls. This finding has been reported by several other groups as well (38-40). Barwick et al. published a case control study that involved 151 patients with AAA and 159 controls, and reported no difference in FDG uptake or SUVmax between those with AAA and controls (23). Several other studies have looked at the relationship between FDG uptake and recent AAA expansion (36,41-44). Whether FDG uptake in the aortic wall in AAA holds clinical meaning remains to be fully clarified. Large, prospective studies will need to be performed to resolve the equivocal results from prior studies.
Novel molecular radiotracers on the horizon
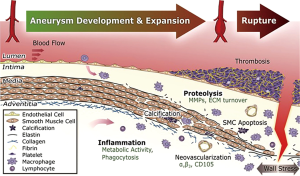
AAs are a complex microenvironment involving several biologic pathways, with increasing evidence that inflammation is important in both abdominal and thoracic aneurysm pathophysiology. Over the past few years, significant advances have been made in the field of fluorination chemistry and other radiotracer developmental technology, whereby ongoing efforts to synthesize PET-compatible novel tracers directed at various inflammatory processes are underway (see Figure 2) (45). For example, tracers targeting endothelial cell adhesion molecules, MMPs, apoptotic caspases, mitochondrial translocator protein (TSPO), integrin receptor, and apolipoprotein E are but a few being investigated (see Table 2) (51-54).
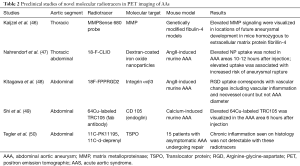
Full table
Several proteases have been identified as important modulators of vessel wall integrity especially with regards to collagen and elastin fibers, and thus may mediate AA growth and rupture. One family of such proteases are the MMP. In particular, some animal studies have used a protease-activated near-infrared fluorescence probe, such as MMPSense 680, to monitor and quantify MMP activity. These studies have documented upregulated MMP activity within AA. In a study using control mice, mice homozygous for the fibulin-4 reduced-expression allele [fibulin-4 (R/R)] showed an enlarged ascending aorta (46). These mice demonstrated significant increase in the presence of MMP-9. Even slight decrease in expression of fibulin-4 in the heterozygous fibulin-4 (+/R) mice showed mild aneurysm formation. In addition, it was shown that MMP levels are elevated prior to overt enlargement in aortic lumen size, and that higher MMP levels predict increased aneurysm progression (46). Pilot clinical studies are needed to determine if greater PET signals reflecting increased MMP levels predict human aneurysm progression.
Arginine-glycine-aspartate (RGD) peptide-based radiotracers targeting integrin alpha-v Beta-3 have been studied in AAA. Integrin alpha-v Beta-3 is upregulated in proliferating macrophages, endothelial cells, and vascular smooth muscle cells (see Figure 3) (48,55-57). In a study using angiotensin II (AngII)-induced murine AAA, an 18F-labeled RGD compound (18F-FPPRGD2), was used to detect vascular changes including vascular inflammation and higher neovessel count. This study demonstrated a correlation between RGD-based radiotracer uptake and vascular changes (vascular inflammation and neovessel count) but not with AAA diameter (48). Similar to some clinical studies, aortic inflammation has been identified in both existing aneurysm and normal-caliber segments, whereby in the latter scenario, inflammation appears to provide additional information to aortic size. Longitudinal studies to determine if inflamed normal diameter segments are predisposed to enlargement need to be performed.
Fab-based molecular radiotracers have also been studied in AAA such as copper-64-labeled CD105 fab antibody fragment. CD105, also called endoglin, is a membrane glycoprotein that interacts with transforming growth factor-beta, an important modulator of AAA formation. Upregulation of CD105 is found in endothelial cells of neovessels and macrophages. A study using a murine calcium-induced AAA, increased PET uptake of the copper-64-labeled CD105 fab antibody fragment radiotracer was noted in the abdomen in the AAA area 6 hours post-injection (49). However, its biological significance is yet to be determined. A limitation of this tracer is the high residual blood pool activity (8% injected dose per gram at 24 hours); often seen with Fab-based radiotracers (58).
TSPO is a mitochondrial protein associated with cholesterol transport and immunomodulation. Radiotracers targeting TSPO, 11C-PK11195 and 11C-d-deprenyl, have been studied in various disorders of systemic inflammation including atherosclerosis and aneurysm. A study looking at the use of these tracers in AAA compared to disorders of systemic inflammation did not show a significant difference between groups. This negative results may have been attributed to the so-called control arm (i.e., systemic inflammatory disorders), which are known to harbor higher degree of arterial inflammation, thereby minimizing the ability to determine a meaningful difference (45,50). With the advent of recognition that inflammation plays a role in TAA, a recent study has shown increased TSPO in the context of TAA (Singh et al.), namely in MFS—a prototype genetically-medicated TAA disorder. This study is small in size and no longitudinal data are yet available.
Other PET radiotracers that have been utilized in AA imaging include nanoparticles-based agents such as fluorine-18-labelled dextran-coated iron oxide nanoparticles (18F-CLIO). In an experimental model with apoE–/– mice, AngII was injected into experimental mice, creating both thoracic and AAA. PET imaging 10 to 12 hours after injection of this radiotracer noted higher uptake in the aneurysmal thoracic and abdominal aortas than in the control mice aortas. In addition, high uptake in early images was associated with higher risk of aneurysm growth and rupture. A limitation of nanoparticle-based radiotracers is their long circulation time mandating delayed imaging to clear blood pool activity. This may be addressed with smaller nanoparticle radiotracers that are cleared more rapidly (47).
Future of clinical imaging of AA
As hybrid PET-CT camera systems continue to mature into an important diagnostic tool, the integration of MRI into PET scanners has surfaced and offers the advance of superior high spatial resolution. Multimodal imaging combines strengths borne from each individual modality and offers valuable integration of molecular, physiologic, and anatomic information. Simultaneous image acquisition with PET and MRI seamlessly co-registers acquired images from both modalities, thereby limiting misalignment and partial volume effects with shorter scan times (59). In addition, PET-MRI hybrid imaging permits synchronized evaluation of multiple physiologic and disease processes by incorporating complementary molecular imaging probes (60,61).
Conclusions
Molecular imaging afforded by hybrid PET imaging systems has emerged as a powerful diagnostic tool to identify potentially high-risk AA. The early identification of these aneurysms is critical to effectuating appropriate care to at risk populations and to lower the risk profile of patients. The road ahead for noninvasive imaging of AA is promising, and with continual efforts dedicated to refining the imaging techniques and performance of prospective clinical studies, the integration of these robust imaging modalities into clinical decision algorithms is promising.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jondeau G, Detaint D, Tubach F, et al. Aortic event rate in the Marfan population: a cohort study. Circulation 2012;125:226-32. [Crossref] [PubMed]
- Pearson GD, Devereux R, Loeys B, et al. Report of the National Heart, Lung, and Blood Institute and National Marfan Foundation Working Group on research in Marfan syndrome and related disorders. Circulation 2008;118:785-91. [Crossref] [PubMed]
- Parish LM, Gorman JH, Kahn S, et al. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg 2009;35:941-5; discussion 945. [Crossref] [PubMed]
- Trimarchi S, Jonker FH, Hutchison S, et al. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg 2011;142:e101-7. [Crossref] [PubMed]
- Kuzmik GA, Sang AX, Elefteriades JA. Natural history of thoracic aortic aneurysms. J Vasc Surg 2012;56:565-71. [Crossref] [PubMed]
- Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg 2009;37:149-59. [Crossref] [PubMed]
- Detaint D, Michelena HI, Nkomo VT, et al. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: a comparative study with Marfan syndrome and degenerative aortopathy. Heart 2014;100:126-34. [Crossref] [PubMed]
- Weinsaft JW, Devereux RB, Preiss LR, et al. Aortic dissection in patients with genetically mediated aneurysms: incidence and predictors in the gentac registry. J Am Coll Cardiol 2016;67:2744-54. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/ SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology,American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons,and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-129. [Crossref] [PubMed]
- Criado FJ. Aortic dissection: a 250-year perspective. Tex Heart Inst J 2011;38:694-700. [PubMed]
- White RA, Miller DC, Criado FJ, et al. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg 2011;53:1082-90. [Crossref] [PubMed]
- Endograft repair of acute aortic dissection: Promises and challenges. Available online: http://search.proquest.com/openview/0c537f3c32416f8d02ff28137311b671/1?pq-origsite=gscholar&cbl=29910
- Ramanath VS, Oh JK, Sundt TM, et al. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc 2009;84:465-81. [Crossref] [PubMed]
- Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med 2013;159:411-20. [Crossref] [PubMed]
- Camici P, Araujo LI, Spinks T, et al. Increased uptake of 18F-fluorodeoxyglucose in postischemic myocardium of patients with exercise-induced angina. Circulation 1986;74:81-8. [Crossref] [PubMed]
- Björnheden T, Levin M, Evaldsson M, et al. Evidence of hypoxic areas within the arterial wall in vivo. Arterioscler Thromb Vasc Biol 1999;19:870-6. [Crossref] [PubMed]
- Evans WH, Karnovsky ML. The biochemical basis of phagocytosis. IV. Some aspects of carbohydrate metabolism during phagocytosis. Biochemistry 1962;1:159-66. [Crossref] [PubMed]
- Hyafil F, Cornily JC, Rudd JH, et al. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J Nucl Med 2009;50:959-65. [Crossref] [PubMed]
- Zhao QM, Feng T, Zhao X, et al. Imaging of atherosclerotic aorta of rabbit model by detection of plaque inflammation with fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Chin Med J 2011;124:911-7. [PubMed]
- Tawakol A, Migrino R, Hoffmann U, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol 2005;12:294-301. [Crossref] [PubMed]
- Hag AM, Pedersen SF, Christoffersen C, et al. (18)F-FDG PET imaging of murine atherosclerosis: association with gene expression of key molecular markers. PLoS ONE 2012;7:e50908. [Crossref] [PubMed]
- Nchimi A, Cheramy-Bien JP, Gasser TC, et al. Multifactorial relationship between 18F-fluoro-deoxy-glucose positron emission tomography signaling and biomechanical properties in unruptured aortic aneurysms. Circ Cardiovasc Imaging 2014;7:82-91. [Crossref] [PubMed]
- Barwick TD, Lyons OT, Mikhaeel NG, et al. 18F-FDG PET-CT uptake is a feature of both normal diameter and aneurysmal aortic wall and is not related to aneurysm size. Eur J Nucl Med Mol Imaging 2014;41:2310-8. [Crossref] [PubMed]
- Kuehl H, Eggebrecht H, Boes T, et al. Detection of inflammation in patients with acute aortic syndrome: comparison of FDG-PET/CT imaging and serological markers of inflammation. Heart 2008;94:1472-7. [Crossref] [PubMed]
- Blockmans D, Coudyzer W, Vanderschueren S, et al. Relationship between fluorodeoxyglucose uptake in the large vessels and late aortic diameter in giant cell arteritis. Rheumatology (Oxford) 2008;47:1179-84. [Crossref] [PubMed]
- Truijers M, Kurvers HA, Bredie SJ, et al. In vivo imaging of abdominal aortic aneurysms: increased FDG uptake suggests inflammation in the aneurysm wall. J Endovasc Ther 2008;15:462-7. [Crossref] [PubMed]
- Sakalihasan N, Van Damme H, Gomez P, et al. Positron emission tomography (PET) evaluation of abdominal aortic aneurysm (AAA). Eur J Vasc Endovasc Surg 2002;23:431-6. [Crossref] [PubMed]
- Fillinger MF, Racusin J, Baker RK, et al. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: Implications for rupture risk. J Vasc Surg 2004;39:1243-52. [Crossref] [PubMed]
- Kent KC, Zwolak RM, Jaff MR, et al. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004;39:267-9. [Crossref] [PubMed]
- Hong H, Yang Y, Liu B, et al. Imaging of Abdominal Aortic Aneurysm: the present and the future. Curr Vasc Pharmacol 2010;8:808-19. [Crossref] [PubMed]
- Kadoglou NP, Liapis CD. Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr Med Res Opin 2004;20:419-32. [Crossref] [PubMed]
- Jacob T, Ascher E, Hingorani A, et al. Initial steps in the unifying theory of the pathogenesis of artery aneurysms. J Surg Res 2001;101:37-43. [Crossref] [PubMed]
- Nordon IM, Hinchliffe RJ, Loftus IM, et al. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011;8:92-102. [Crossref] [PubMed]
- Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med 2014;371:2101-8. [Crossref] [PubMed]
- Giles KA, Pomposelli F, Hamdan A, et al. Decrease in total aneurysm-related deaths in the era of endovascular aneurysm repair. J Vasc Surg 2009;49:543-50; discussion 550. [Crossref] [PubMed]
- Reeps C, Essler M, Pelisek J, et al. Increased 18F-fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg 2008;48:417-23; discussion 424. [Crossref] [PubMed]
- Tegler G, Ericson K, Sörensen J, et al. Inflammation in the walls of asymptomatic abdominal aortic aneurysms is not associated with increased metabolic activity detectable by 18-fluorodeoxglucose positron-emission tomography. J Vasc Surg 2012;56:802-7. [Crossref] [PubMed]
- Palombo D, Morbelli S, Spinella G, et al. A positron emission tomography/computed tomography (PET/CT) evaluation of asymptomatic abdominal aortic aneurysms: another point of view. Ann Vasc Surg 2012;26:491-9. [Crossref] [PubMed]
- Marini C, Morbelli S, Armonino R, et al. Direct relationship between cell density and FDG uptake in asymptomatic aortic aneurysm close to surgical threshold: an in vivo and in vitro study. Eur J Nucl Med Mol Imaging 2012;39:91-101. [Crossref] [PubMed]
- Morbelli S, Ghigliotti G, Spinella G, et al. Systemic vascular inflammation in abdominal aortic aneurysm patients: a contrast-enhanced PET/CT study. Q J Nucl Med Mol Imaging 2014;58:299-309. [PubMed]
- Kotze CW, Menezes LJ, Endozo R, et al. Increased metabolic activity in abdominal aortic aneurysm detected by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT). Eur J Vasc Endovasc Surg 2009;38:93-9. [Crossref] [PubMed]
- Morel O, Mandry D, Micard E, et al. Evidence of Cyclic Changes in the Metabolism of Abdominal Aortic Aneurysms During Growth Phases: 18F-FDG PET Sequential Observational Study. J Nucl Med 2015;56:1030-5. [Crossref] [PubMed]
- 18F-FDG Aortic Aneurysm Uptake on PET/CT is Inversely Associated with Growth Rate. Available online: http://discovery.ucl.ac.uk/1326173/
- Kotze CW, Rudd JH, Ganeshan B, et al. CT signal heterogeneity of abdominal aortic aneurysm as a possible predictive biomarker for expansion. Atherosclerosis 2014;233:510-7. [Crossref] [PubMed]
- Toczek J, Meadows JL, Sadeghi MM. Novel molecular imaging approaches to abdominal aortic aneurysm risk stratification. Circ Cardiovasc Imaging 2016;9:e003023. [Crossref] [PubMed]
- Kaijzel EL, van Heijningen PM, Wielopolski PA, et al. Multimodality imaging reveals a gradual increase in matrix metalloproteinase activity at aneurysmal lesions in live fibulin-4 mice. Circ Cardiovasc Imaging 2010;3:567-77. [Crossref] [PubMed]
- Nahrendorf M, Keliher E, Marinelli B, et al. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscler Thromb Vasc Biol 2011;31:750-7. [Crossref] [PubMed]
- Kitagawa T, Kosuge H, Chang E, et al. Integrin-targeted molecular imaging of experimental abdominal aortic aneurysms by (18)F-labeled Arg-Gly-Asp positron-emission tomography. Circ Cardiovasc Imaging 2013;6:950-6. [Crossref] [PubMed]
- Shi S, Orbay H, Yang Y, et al. PET Imaging of Abdominal Aortic Aneurysm with 64Cu-Labeled Anti-CD105 Antibody Fab Fragment. J Nucl Med 2015;56:927-32. [Crossref] [PubMed]
- Tegler G, Sörensen J, Ericson K, et al. 4D-PET/CT with [(11)C]-PK11195 and [(11)C]-(D)-deprenyl does not identify the chronic inflammation in asymptomatic abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2013;45:351-6. [Crossref] [PubMed]
- Probes for Non-invasive Matrix Metalloproteinase-targeted Imaging with PET and SPECT. Available online: http://www.ingentaconnect.com/content/ben/cpd/2013/00000019/00000025/art00011
- Limpachayaporn P, Schäfers M, Schober O, et al. Synthesis of new fluorinated, 2-substituted 5-pyrrolidinylsulfonyl isatin derivatives as caspase-3 and caspase-7 inhibitors: nonradioactive counterparts of putative PET-compatible apoptosis imaging agents. Bioorg Med Chem 2013;21:2025-36. [Crossref] [PubMed]
- Gaemperli O, Shalhoub J, Owen DR, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J 2012;33:1902-10. [Crossref] [PubMed]
- Kawachi E, Uehara Y, Hasegawa K, et al. Novel Molecular Imaging of Atherosclerosis With Gallium-68-Labeled Apolipoprotein A-I Mimetic Peptide and Positron Emission Tomography. Circ J 2013;77:1482-9. [Crossref] [PubMed]
- Meoli DF, Sadeghi MM, Krassilnikova S, et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest 2004;113:1684-91. [Crossref] [PubMed]
- Sadeghi MM, Krassilnikova S, Zhang J, et al. Detection of injury-induced vascular remodeling by targeting activated alphavbeta3 integrin in vivo. Circulation 2004;110:84-90. [Crossref] [PubMed]
- Razavian M, Marfatia R, Mongue-Din H, et al. Integrin-targeted imaging of inflammation in vascular remodeling. Arterioscler Thromb Vasc Biol 2011;31:2820-6. [Crossref] [PubMed]
- Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011;473:308-16. [Crossref] [PubMed]
- Pichler BJ, Judenhofer MS, Pfannenberg C. Multimodal imaging approaches: PET/CT and PET/MRI. Handb Exp Pharmacol 2008.109-32. [Crossref] [PubMed]
- Lee WW, Marinelli B, van der Laan AM, et al. PET/MRI of inflammation in myocardial infarction. J Am Coll Cardiol 2012;59:153-63. [Crossref] [PubMed]
- Majmudar MD, Keliher EJ, Heidt T, et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 2013;127:2038-46. [Crossref] [PubMed]


