Childhood bronchial tuberculosis: report of one case and literature review
Bronchial tuberculosis (BTB), previously known as endobronchial tuberculosis (EBTB), refers to the tuberculosis that occurs in trachea or bronchus (1). BTB is mainly seen in young and mid-aged adults but is relatively rare in children. Here we report a pediatric patient with BTB that was confirmed by bronchoscopy in our hospital.
Case report
The 9-year-old boy experienced frequent coughing with phlegm five years ago, which as accompanied with chest tightness and wheezing when the symptom became severer. He had been repeatedly treated in the department of pediatrics of another hospital, during which chest radiography showed no obvious abnormality. He was suspected to be with “bronchial asthma”. After repeated anti-infection treatment and long-term administration of fluticasone propionate and salmeterol inhalation powder (Seretide®), the cough was somehow mitigated. Three months before his admission to our hospital, he suffered from paroxysmal cough again. The cough was accompanied by yellow phlegm, which was hard to cough up. Hemoptysis or bloody sputum was not observed. Night sweats were present. Chest tightness and wheezing became more obvious after physical activities. Since he had previously been diagnosed as “bronchial asthma”, anti-infection and asthma-resolving treatment was provided again and no special attention was paid. About half month before his admission to our hospital, he underwent adenoidectomy and radiofrequency ablation for both inferior nasal meatus due to “adenoidal hypertrophy and chronic sinusitis”, and the post-operative pathology prompted “nasopharyngeal tuberculosis”. Then, chest X-ray examination was performed, which showed that “there is a triangle small opacity in the left lower lung behind the heart shadow and the air volume increases in the left lung”. Purified protein derivative (PPD) (5 IU) showed moderately positive results (15 mm × 14 mm). He was then transfered to our hospital on suspicion of “lung tuberculosis”. Detection for serum anti-Mycobacterium tuberculosis antibody (Western blotting), serum anti-Mycobacterium tuberculosis lipoarabinomannan (LAM) antibody, and serum anti-Mycobacterium tuberculosis 38 KD antibody (protein chip method) showed positive results. He was then admitted to our hospital on suspicion of “atelectasis of the left lower lobe, with suspected bronchial tuberculosis”. During his disease course, the patient had no obvious fever or chest pain, his food intake, sleep, and defecation were basically normal, and the body weight showed no remarkable change. He was born healthy. His parents denied any other previous disease except for “bronchial asthma”. Also, he had no previous disease of drug/food allergy. He received all of his immunizations on schedule. Physical examination showed: body temperature, 37 °C; pulse, 106 times/min; respiration, 24 times/min; and blood pressure, 96/66 mmHg. His development was normal. No rash or purpura occurs in the skin or mucous membrane. No superficial lymph node was palpable throughout the body. His chest was symmetrical. Breathing movement of the left side weakened and there was left lower lobe percussion dullness. A localized wheeze was heard in the left lung, and coarse breath sounds could be heard in the other lung. No wet or dry rale was heard. Examinations of heart, abdomen, limbs, and nervous system showed no obvious abnormality.
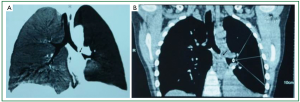
According to the recommended protocol, the patient received anti-TB treatment using “isoniazid 300 mg qd, rifampicin 300 mg qd, ethambutol 500 mg qd, and pyrazinamide 250 mg bid”, anti-infection treatment using “ticarcillin/clavulanate potassium 3,200 mg bid, iv”, prophylactic liver-protecting treatment using “glutathione, 1,200 mg, qd, iv”, phlegm-resolving treatment using “ambroxol 30 mg, qd, iv”, and treatment for relieving airway hyperresponsiveness using “ketotifen 1 mg qd”. Chest CT and three-dimensional airway reconstruction showed atelectasis of left lower lobe, increased air volume of the left upper lung, left deviation of the mediastinum, bronchiolitis obliterans in the left lower lobe, and narrowing of the left upper lobe bronchus (Figures 1A,B, and 2). All the three sputum samples were negative for the bacteria. Lung function: the patient had mild restrictive ventilatory dysfunction, decreased diffusing capacity, and moderately increased residual volume/total lung capacity ratio; anyway, the airway resistance was within the normal range. T lymphocyte subsets: the CD4/CD8 ratio was 2.05, and no other abnormality was found. Blood lipids: the cholesterol was 8.67 mmol/L and the low-density lipoprotein cholesterol was 3.34 mmol/L, prompting the presence of hyperlipidemia. Other routine tests including routine blood tests, C-reactive protein, erythrocyte sedimentation rate, liver/kidney functions, serum hepatitis B markers, hepatitis C antibody, anti-HIV antibody, and blood autoantibody profiles showed no abnormalities. Cardiac, and digestive/urinary ultrasound showed normal results. Bronchoscopy showed that the lumen of the left main bronchus became narrow and the mucosa had severe congestion and edema. In addition, because the lumen was clogged by the necrotic tissues, the bronchoscope could not enter (Figure 3A). With the assistance of bronchoscope, after the secretions were suctioned, interventional treatment with isoniazid 200 mg and amikacin 400 mg were provided weekly. After one month of treatment, the necrotic tissues around the wall of the left main bronchus disappeared; however, the mucosa still had severe congestion and edema and the lumen remained narrow, and therefore the endoscope still could not reach the distal end (Figure 3B). Considering the fact that the narrow left main bronchus affected the airway patency and drainage, bronchofiberoscopic ballon dilation was performed once, which showed good effectiveness. The left main bronchus was enlarged, allowing the smooth access of the bronchoscope. Except that the opening of apical segment remained narrow and inaccessible, the lumen of other segments basically became patent (Figure 3C). Chest CT examination during the follow-up examination one month later showed that the atelectasis of the left lower lobe was reversed, and TB lesions in the left lung were absorbed, and the air volume of the left upper lung returned normal (Figure 2B).
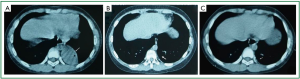
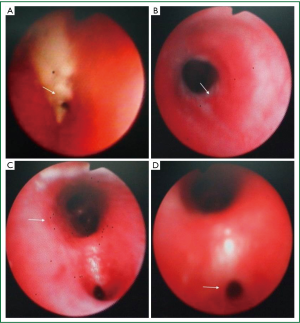
The initial sputum culture had confirmed that the growth of sensitive Mycobacterial tuberculosis; after 9 months of follow-up, the symptoms have completely resolved. After 6 months of treatment, bronchoscopy showed that only the posterior segment was somehow narrow, and the mucous membrane of the other segments of the bronchus was basically stable, and the lumena were patent (Figure 3D). After 9 months of treatment, chest CT showed that only a few fibrotic shadows were visible in the left lower lobe (Figure 2C). Sputum test for Mycobacterium tuberculosis showed negative result.
Discussion
The early clinical manifestations of childhood BTB are often nonspecific. Although irritable cough accompanied with localized wheezing may be the important features of BTB, they are not frequently seen. Chest X-ray and even CT often show normal results in the early stage (2). Clinically, BTB is often underdiagnozed, or misdiagnosed as bronchitis, bronchial asthma, cough variant asthma, or bronchiectasis. Delayed treatment may result in tracheobronchial stenosis, atelectasis, impaired lung function, and even systemic tuberculosis, posing serious impact on children’s health and development. In our current case, the pediatric patient was presented due to repeated cough and breathing difficulty. His early chest X-ray showed no obvious abnormality. Therefore, he was diagnosed as “bronchial asthma” and received symptomatic treatment. Finally, he developed nasopharyngeal TB, left tracheal BTB, and atelectasis of the left lower lobe. Therefore, early diagnosis and timely treatment are particularly important. PPD test showed positive result in this patient, which provided solid evidence for the clinical diagnosis of TB. BTB was confirmed based on the findings of chest CT, 3D airway reconstruction, and bronchoscopy. Therefore, pediatric patients with long-term chronic cough, repeated localized or one-sided wheeze, and recurrent symptoms after anti-infection and anti-asthma treatment but without abnormal findings in chest X-ray should be cautiously managed in clinical settings, particularly in the department of respiratory medicine. The possibility of BTB should be early considered to avoid any potential obstructive pulmonary disease or even atelectasis. Since many parents and physicians are reluctant to arrange CT or bronchoscopy for the children, PPD test may be performed firstly. As a simple, affordable, and non-invasive tool, PPD test can play an important role for TB diagnosis in children, particularly for those whose PPD status has recently shifted from negative to positive (>10 mm × 10 mm). Chest CT and 3D airway reconstruction are recommended for PPD-positive children with chronic cough. In most children, these two imaging modes can clearly reveal the bronchial stenosis, the obstructed bronchial lumen, and even the swollen pulmonary hilum/mediastinal lymph nodes, and thus provide vital information for BTB diagnosis (3). However, the confirmation of diagnosis is still based on the bronchoscopic findings.
Bronchoscopy and biopsy are the most important approaches to the confirmation of BTB, and the bronchoscopic findings of BTB may vary. In 2009, Tang et al. (4) proposed the diagnostic criteria of BTB: (I) bronchoscopically visible bronchial lesions; (II) responsive to anti-TB treatment; (III) bronchial washing smear for acid-fast Mycobacterium tuberculosis was positive, or acid-fast Mycobacterium tuberculosis was detected in BALF; (IV) sputum smear for acid-fast mycobacterium was positive, or Mycobacterium tuberculosis grows in the culture medium; and (V) bronchial biopsy confirmed the existence of BTB. The diagnosis can be confirmed if a patient meets the criteria I, II, and III (or IV), or if a patient meets the criteria I and V. Chung et al. (5) classified forms of endobronchial tuberculosis (EBTB) into seven subtypes by bronchoscopic finding: (I) nonspecific bronchitic; (II) edematous-hyperemic; (III) actively caseating; (IV) ulcerative; (V) granular; (VI) tumorous; and (VII) fibrostenotic. Based on literature review, a Chinese-version expert consensus in 2012 divided the bronchoscopic findings of BTB into the following six forms: (I) inflammatory infiltration; (II) ulcers and necrosis; (III) inflammatory granulation; (IV) scarring and stenosis; (V) wall softening; and (VI) lymphatic fistula. If two or more different lesions co-exist, record the main lesion (6).
In our current case, the bronchoscopic findings of BTB included four forms: inflammatory infiltration, necrosis, inflammatory granulation, and scarring and stenosis, with caseous necrosis being the main lesion. Due to the difficulty in the early diagnosis of BTB, the disease usually has progressed into the stages of caseous necrosis and/or granulation, and the patient may rapidly develop tracheobronchial stenosis (5). The prognosis of childhood BTB is highly depended on early diagnosis and timely treatment. The clinical management of BTB is based on the systemic anti-TB treatment, along with the application of bronchoscopy and its interventional therapies (7). The interventional bronchoscopy include: local injection of anti-TB drugs (e.g., isoniazid and amikacin), cryotherapy, balloon dilatation, microwave, argon plasma congulation, and high-frequency electrotome (8). For patients who are non-responsive to the combination of the above therapies and experience repeated obstructive pneumonia and atelectasis, temporary stent placement may be cautiously considered. Notably, the long-term efficacy of stent placement remains unknown, and its complications are also problematic. Clinical practices in recent years have shown that microwave, argon plasma congulation, high-frequency electrotome, stent placement are not feasible for TB and some other benign bronchial lesions because they may result in severe granulation and thus cause tracheobronchial restenosis. In our present case, the patient received local injection of anti-TB drugs under bronchoscope on the basis of systemic anti-TB treatment; by doing so, we timely eliminated caseous necrosis or inflammatory granulation, and thus avoid the repeated occurence of obstructive pneumonia or atelectasis. The bronchial scars and stenosis were timely treated with bronchoscopic balloon dilatation, which effectively improved the airway ventilation, improved the treatment efficacy, and reduced the complications.
The incidence of childhood BTB has increased in recent years. However, due to its non-specific clinical manifestations, childhood BTB is often underdiagnosed or misdiagnosed; as a result, it is often not treated timely, resulting in severe complications, which severely affect the children’s health and development. It has been reported that 41.7-43.0% of pediatric patients with active pulmonary TB were accompanied with BTB (9). In addition, some children with simple BTB (i.e., no remarkably active TB lesion was visible inside the lungs) are more likely to be underdiagnozed or misdiagnozed. However, its prevalence remains unclear. In summary, pediatric patients with long-term chronic cough must be cautiously managed. Any potential BTB must be checked as early as possible. Interventional bronchoscopy must be performed earlier to provide the maximal protection of the bronchus and lungs and minimize the occurrence of complications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Xue Q, Wang N, Xue X, et al. Endobronchial tuberculosis: an overview. Eur J Clin Microbiol Infect Dis 2011;30:1039-44. [PubMed]
- Cakir E, Uyan ZS, Oktem S, et al. Flexible bronchoscopy for diagnosis and follow up of childhood endobronchial tuberculosis. Pediatr Infect Dis J 2008;27:783-7. [PubMed]
- Kashyap S, Mohapatra PR, Saini V. Endobronchial tuberculosis. Indian J Chest Dis Allied Sci 2003;45:247-56. [PubMed]
- Tang SJ, Xiao HP, Hu HL, et al. Clinical characteristic, diagnostic standard and types of endobronchial tuberculosis: An analysis of 278 cases. Chinese Journal of Clinicians 2009;3:32-40.
- Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117:385-92. [PubMed]
- Tuberculosis branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of bronchial tuberculosis of trachea. Clin J Tuberc Respir Dis 2012;35:581-7.
- Um SW, Yoon YS, Lee SM, et al. Predictors of persistent airway stenosis in patients with endobronchial tuberculosis. Int J Tuberc Lung Dis 2008;12:57-62. [PubMed]
- Li Q. eds. Respiratory Endoscopy. Shanghai: Shanghai Science and Technology Press, 2003.
- Tagarro García A, Barrio Gómez de Agüero MI, Martínez Carrasco C, et al. Fiberoptic bronchoscopy in childhood endobronchial tuberculosis. An Pediatr (Barc) 2004;61:314-9. [PubMed]


