Transcatheter mitral valve replacement: an evolution of a revolution
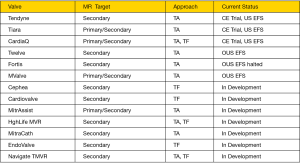
Mitral regurgitation (MR) is the most prevalent valve disease in the United States, affecting 6% of people over 75 years of age (1). Surgical intervention is indicated in patients with severe MR and symptoms of heart failure despite optimal medical therapy, and for patients with reduced left ventricle (LV) systolic function, atrial fibrillation or pulmonary hypertension (2). However, many of these patients are denied surgery due to their excess surgical risk (3). The unmet clinical need in these patients coupled with the steady progress of transcatheter aortic valve replacement (TAVR) have inspired an explosion of clinical investigations aiming at a matching success in transcatheter therapies for mitral valve (MV) diseases (4). To date, the MitraClip (Abbott vascular, Santa Clara, CA) is the only transcatheter MV system that is approved for commercial use. MitraClip has demonstrated feasibility, safety, and efficacy in reducing MR through a decade of clinical investigations and clinical experience in over 40,000 patients (5,6). Despite the proven benefits, MitraClip is limited to a small proportion of anatomically and clinically suitable patients. The need for a transcatheter treatment modality with wider applicability and the more predictable reduction of MR, led to a substantial investment in the transcatheter mitral valve replacement (TMVR) field. Currently, there are at least 30 dedicated TMVR systems in development, but only a handful that reached early feasibility studies (EFS) in humans (Figure 1). In this manuscript, we aim to:
- Summarize the challenges of TMVR;
- Describe the unique feature of the Tendyne TMVR system (Abbott, Roseville, Minnesota);
- Discuss the recently reported initial data of the Tendyne EFS.
Challenges of TMVR
The achievements of TAVR are viewed as the benchmark for comparable transcatheter systems aiming to treat other valves including the MV. Unfortunately, the complex pathoanatomy of the MV among other factors, posed several unique challenges to the field of TMVR:
- Patient’s population: compared with aortic stenosis (AS) patients, those with MR are younger. Therefore, emerging TMVR systems need to compete against established standards of care in a younger and relatively healthier population than the TAVR population.
- Mitral disease etiology: several etiologies are implicated in the development of MR with significant overlaps between them. This is contrary to the common degenerative calcific etiology of AS in patients undergoing TAVR.
- Benefit of therapy: compared with the substantial advantage of aortic valve replacement for patients with AS, the benefit of MV repair or replacement in patients with secondary MR (currently the main target population of TMVR trials) is less pronounced. In addition, MV repair is currently preferred over replacement in the surgical community and a wide application of TMVR may therefore be counterintuitive (7).
- Device specific challenges:
- The mitral annulus is asymmetrical and has no stable calcified structure for anchoring of transcatheter valves;
- The radial stiffness of the bioprosthesis has to be carefully optimized to resist frame fracture, prevent embolization and avoid perforation of adjacent structures;
- Delivery rout: for TMVR systems designed for transapical delivery such as the Tendyne valve, the challenges of transapical access (learning curve, long-term effects of ventricular function, access site bleeding, etc.) have to be overcome (8). Similarly, other issues (precision of valve deployment, iatrogenic atrial septal defect, etc.) needs to be addressed before the transseptal rout for TMVR becomes mainstream (9,10);
- The risk of left ventricular outflow (LVOT) obstruction, due to the bulkiness of the device, especially with valve designs that do not capture the native mitral leaflets (11);
- Contrary to para-aortic regurgitation, mild degrees of paravalvular regurgitation may not be acceptable as paravalvular leak is not well tolerated in the mitral position (12,13). Therefore, optimal TMVR have to be designed to mitigate the risk of paravalvular regurgitation.
- Concerns related to the durability and the potential thrombogenicity of the bioprosthesis will require a decade long investigations to be adequately addressed (14,15).
Tendyne transcatheter MV system
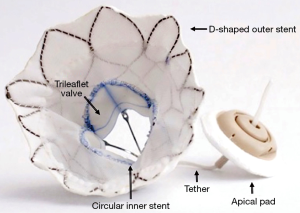
The current design of the Tendyne bioprosthetic MV system is delivered in a 34-F sheath via a transapical approach. The valve consists of two Nitinol self-expanding joined stents: (I) an inner one-size circular stent, onto three porcine pericardial leaflets are sewn; and (II) an outer D-shaped stent that conforms to the shape of the mitral annulus and is covered with a polyethylene terephthalate (PET) fabric cuff that provides the sealing surface within the native annulus (Figure 2). The outer stent frame is available in 13 different sizes. The inner and outer stent are joined together to form the prosthesis, which is connected to a pad that rests on the epicardial surface via braided fiber tether. The tether is designed to stabilize the valve, and possibly enhance positive remodeling (16). The valve does not have a mechanism to capture the native MV leaflets, and the risk of LVOT obstruction has to be carefully assessed especially in patients with narrow LVOT or those with an elongated anterior mitral leaflet. The valve is fully repositionable and retrievable. Compared with the first generation valve, the Tendyne 2.0 valve comes with a smaller inner stent size, and therefore a small valve area (effective orifice area of the Tendyne 2. is 2.0 vs. 3.2 cm2 for the first generation valve), a shorter frame and a more tapered outflow to minimize the risk of LVOT obstruction. In addition, two major refinements of the delivery system are underway; iTendyne, a novel system designed for transseptal delivery of the bioprosthesis is in the investigational phase, and the Gen-II transapical delivery system featuring a wheel rotation deployment mechanism, a pad positioning tool, and tower loading for a much smoother and intuitive deployment is in its final production stages.
Tendyne early feasibility trial
Muller et al recently reported the results of the EFS of the Tendyne MV System, the largest published TMVR experience to date (14). In this study, 30 symptomatic patients with severe MR (+4 in 93%) who are at high risk for surgical interventions were treated at 8 sites. Patients mean age was 75.6±9.2 years, 83% were males, and 47% had prior coronary artery bypass grafting. The mean Society of Thoracic Surgery predicted risk of mortality was 7.3%. The etiology of MR was secondary, primary and mixed in 77%, 10% and 13% of patients, respectively. Left ventricular function systolic function was diminished in the majority of patients (<50% in 59% of patients).
The valve was successfully implanted in 93% of patients. Three patients required intraoperative retrieval of the device due to under-sizing, LVOT obstruction and non-coaxiality of the access with the mitral annulus. A larger size valve was implanted without complications in the first patients, but the valves were retrieved without replacement in the other two patients. Mild paravalvular leak was detected after valve deployment in one patient (3.3%). Three patients (10%) required blood transfusion due to access site bleeding. Two third of patients were discharged home, at a mean hospital length of stay of 9.7±5.9 days.
At 30-day follow-up, all-cause mortality occurred in one patient (3.3%) due to hospital-acquired pneumonia. There was no device migration, embolization, or need for mitral surgery. However, two patients had device related dysfunction that was managed medically; one had evidence of valve thrombosis while subtherapeutic on anticoagulation and was treated successfully with intensified anticoagulation, and one had hemolysis requiring blood transfusion related to a presumable paravalvular regurgitation. Three patients (10%) developed new left bundle branch block, although none developed heart block or needed a permanent pacemaker implantation. Four patients (14%) were rehospitalized for heart failure. MR grade was 0 in 96% of patients. Left ventricular end diastolic volume index decreased significantly from 90.1±28.2 mL/m2 at baseline to 72.1±19.3 mL/m2 at 30 days, P=0.0012. Interestingly, mean left ventricular ejection fraction (LVEF) decreased from 47.1%±9.2% to 41.3%±9.5%. There was no change in 6-min walk distance (299.7±210.6 m at baseline vs. 294.4±136.9 m at 30-day follow-up), but the Kansas City Cardiomyopathy Questionnaire quality-of-life score improved from 50.2±23.5 to 64.6±26.3 (P=0.0018).
The primary performance endpoint (successful device implantation and freedom from cardiovascular mortality, stroke, and device dysfunction at 30 days) was achieved in 86.7% of patients. The primary safety endpoint (freedom from cardiovascular mortality, disabling stroke, myocardial infarction, reintervention for valve-related dysfunction, life-threatening bleeding (Bleeding Academic Research Consortium type 2, 3, or 5), and renal failure requiring dialysis at 30 days) was achieved in 83.3% of patients.
The key finding of this study is remarkable: TMVR can be performed safely, with a low risk of procedural death and major adverse events. However, like any EFS, the study leaves us with more questions than answers.
- The mean LVEF fell from 47% to 41%, which warrants further investigations. Although this might be related to the increase in afterload and decrease in preload associated with the reduction in MR, reports of adverse effect of transapical access on LV function in the TAVR literature raise some concerns about a similar issue with transapical TMVR. Compared with transapical TMVR, would TMVR systems utilizing the transseptal rout have a more positive impact on LVEF? Is this drop in LVEF related to the tethering mechanism of the Tendyne valve?
- The consequences of implanting a bulky device in a low-pressure system need to be assessed. In this study one patient developed valve thrombosis on sub-therapeutic anticoagulation, and another patient developed significant hemolysis despite the absence of imaging evidence of paravalvular regurgitation. How frequent will valve thrombosis occur? What is the optimal anticoagulation regimen following TMVR? Does the valve itself cause hemolysis? Is there micro-hemolysis that is undetectable on imaging but is chemically significant with this valve?
- Infrequently occurring adverse events such as (access site bleeding, new conduction abnormalities, and paravalvular regurgitation) need to be further minimized. Are these preventable events?
- Despite the promising results of the current study, the design of a pivotal TMVR trial will be faced with several hurdles:
- Should such a trial include high surgical risk patients with primary MR or just those with secondary MR? If primary MR patients were to be included, would enrollment be an issue given that there is an approved transcatheter system (MitraClip) with proven safety and efficacy to treat these patients?
- What would patients undergoing TMVR be randomized against (medical therapy vs. surgery). If surgery is chosen and the valve is repairable, would these patients be required to have MV replacement despite the possibility of repair? Also, a large proportion of MR patients have concomitant tricuspid regurgitation (TR). Those patients with significant concomitant TR will likely undergo surgical treatment of their TR at the time of MV surgery. What impact would the untreated TR have on the outcomes of the TMVR group?
- What would the primary endpoint for the trial be? If mortality was the chosen primary endpoint, testing non-inferiority or superiority to the control arm might require a very long follow up. This is because MR patients (unlike those with AS) do not have a high short-term mortality, and the adverse impact of their mitral disease is rather seen in the longer-term.
In summary, the excellent short-term outcomes reported in the Tendyne EFS constitute a great first step in the evolving TMVR revolution. The road of TMVR will continue to be a challenging one, but difficult roads often lead to beautiful destinations!
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875-83. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017.2017. [Epub ahead of print]. [PubMed]
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65. [Crossref] [PubMed]
- Taramasso M, Candreva A, Pozzoli A, et al. Current challenges in interventional mitral valve treatment. J Thorac Dis 2015;7:1536-42. [PubMed]
- Feldman T, Kar S, Elmariah S, et al. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. J Am Coll Cardiol 2015;66:2844-54. [Crossref] [PubMed]
- Sorajja P, Mack M, Vemulapalli S, et al. Initial Experience With Commercial Transcatheter Mitral Valve Repair in the United States. J Am Coll Cardiol 2016;67:1129-40. [Crossref] [PubMed]
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53. [Crossref] [PubMed]
- Alkhouli MA, Raybuck BD, Badhwar V. Navigating the s-curve of transapical therapy. J Thorac Cardiovasc Surg 2016;152:781-2. [Crossref] [PubMed]
- Alkhouli M, Rihal CS, Holmes DR Jr. Transseptal Techniques for Emerging Structural Heart Interventions. JACC Cardiovasc Interv 2016;9:2465-2480. [Crossref] [PubMed]
- Alkhouli M, Sarraf M, Zack CJ, et al. Iatrogenic atrial septal defect following transseptal cardiac interventions. Int J Cardiol 2016;209:142-8. [Crossref] [PubMed]
- Khan JM, Rogers T, Schenke WH, et al. Intentional Laceration of the Anterior Mitral Valve Leaflet to Prevent Left Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement: Pre-Clinical Findings. JACC Cardiovasc Interv 2016;9:1835-43. [Crossref] [PubMed]
- Alkhouli M, Sarraf M, Maor E, et al. Techniques and Outcomes of Percutaneous Aortic Paravalvular Leak Closure. JACC Cardiovasc Interv 2016;9:2416-26. [Crossref] [PubMed]
- Sorajja P, Cabalka AK, Hagler DJ, et al. Long-term follow-up of percutaneous repair of paravalvular prosthetic regurgitation. J Am Coll Cardiol 2011;58:2218-24. [Crossref] [PubMed]
- Muller DW, Farivar RS, Jansz P, et al. Transcatheter Mitral Valve Replacement for Patients With Symptomatic Mitral Regurgitation: A Global Feasibility Trial. J Am Coll Cardiol 2017;69:381-91. [Crossref] [PubMed]
- Hudec V, Bena M, Artemiou P, et al. Reversible thrombotic mitral valve stenosis after transcatheter mitral valve replacement (TMVR): Is life-long anticoagulation therapy necessary? J Card Surg 2017;32:190-2. [Crossref] [PubMed]
- Moat NE, Duncan A, Quarto C. Transcatheter mitral valve implantation: Tendyne. EuroIntervention 2016;12:Y75-7. [Crossref] [PubMed]