Expedite recovery from esophagectomy and reconstruction for esophageal squamous cell carcinoma after perioperative management protocol reinvention
Introduction
Cancer is the leading cause of death in Taiwan, and esophageal cancer is ranked number nine among them. There were 2,496 new cases, of which 754 received resection in 2013 (1).
Radical resection and reconstruction for esophageal cancer carry certain risks. The operative time is relatively long; it is also associated with high morbidity. In addition to this, the post-operative course is strenuous, owing to possible respiratory complications, anastomotic leakage, and poor nutritional status. Consequently, hospital stay after surgical treatment for esophageal cancer is often long, leading not only to increased financial burden, but also late return to normal life.
Thus Kehlet and Wilmore’s research in 2002 (2) provided a basis for our study. They introduced a fast-track surgery pathway in order to advance post-operative surgical care, with positive results including post-operative recovery enhancement, reduction of patients’ physiological and psychological stress, and decrease in length of hospital stay (2,3).
As we know, the reduction of hospital expenditure is currently a primary focus for policy on a worldwide scale due to increasing requisite and shortage of resources. With this in mind, how we advance the convalescence of post-esophagectomy patients is an imperative and important issue in all medical centers.
Confident in our mature surgical dexterity, experienced anesthesiology technique and well-trained postoperative care team, in 2012, we reinvented our old protocol for improvement of postoperative recovery of patients who received esophagectomy and reconstruction for esophageal squamous cell carcinoma after detailed interdisciplinary discussions and meetings (Table 1). Video-assisted thoracic surgery (VATS) esophagectomy was also adopted as we have been implementing VATS for pulmonary surgery since 2006. This study is to determine whether the new protocol led to an improvement of patient recovery, including complications, mortality, hospital stay, and financial burden.

Full table
Methods
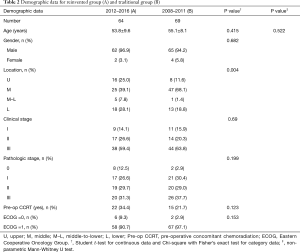
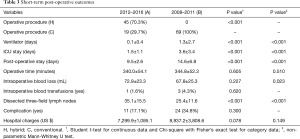
This is a retrospective study. Patients with esophageal squamous cell carcinoma who had received esophagectomy and reconstruction with stomach were reviewed. Data from two groups of patients, categorized according to their treatment period, were collected. Group A patients were cared for according to the recently adopted protocol, starting from January 2012. Up to June 2016, 64 patients were collected. Group B was a group of 69 patients who had been placed on the old protocol between January 2008 and December 2011. All patients had suffered from esophageal squamous cell carcinoma. They all received esophagectomy through thoracotomy or VATS, reconstruction with stomach through laparotomy and feeding jejunostomy. There was no case where the colon was used as an esophageal substitute. Patients’ demographic data and pre-operative ECOG (Eastern Cooperative Oncology Group), tumor location, clinical stage, pathologic stage, pre-operative treatment modality, length of post-operative stay, days in ICU, post-operative ventilator days, operative time, intraoperative blood loss, intraoperative blood transfusions, dissected three-field lymph nodes, complications, 30-day hospital mortality, and medical charges were recorded (Tables 2 and 3).
Full table
Full table
Cessation of smoking for all patients for at least two weeks before operation was mandatory.
All data was obtained as part of routine clinical care from charts so that extra patient consent was waived. The study was approved by IRB [KMUH-IRB-E(II)-20150240].
All clinical and pathologic staging were determined according to the criteria of the 7th Edition of the AJCC cancer staging manual (4).
Surgical procedure
- Hybrid (H)—VATS esophagectomy and Laparotomy reconstruction with stomach.
Patients in Group A were operated on with Hybrid procedure. Twenty-nine point seven percent were converted into thoracotomy esophagectomy owing to severe local tumor adhesions after pre-operative concomitant chemoradiotherapy (CCRT). - Conventional procedure—thoracotomy esophagectomy + laparotomy for stomach tube.
All group B patients received the conventional procedure.
Operative technique
Abdominal procedure
Median laparotomy was made, followed by mobilization of the entire great curvature of the stomach to preserve the right gastroepiploic artery as the pedicle. The short and left gastric vessels were divided. The abdominal esophagus was transected. The gastric conduit was made by stapling the lesser curvature and forming a gastric tube. Kocher’s maneuver was performed when necessary. Abdominal lymphadenectomy and feeding jejunostomy were routinely performed.
Neck procedure
A 5-cm oblique incision was made over the anterior border of the left sternocleidomastoid muscle. The neck esophagus was mobilized to facilitate communication with the right chest. Left neck lymphadenectomy was also carried out with caution so not to injure the left recurrent laryngeal nerve. Gastric tube was pulled up through the substernal route. Cervical anastomoses were all conducted using hand-sewn interrupted mattress suture technique.
Thoracic procedure
The patients were placed in left lateral decubitus position. A right posterolateral thoracotomy incision was made. Mediastinal pleura were incised and the esophagus was separated from the adjacent mediastinal tissues and organs. The azygos venous arcade was routinely divided. Mediastinal lymphadenectomy was also performed. For VATS approach, utility of three 10-mm trocars were placed over fourth intercostal space, anterior axillary line; sixth intercostal space, anterior axillary line, and eighth intercostal space, posterior axillary line. The operative steps were performed in a similar fashion as open thoracotomy. The operative procedure had been shifted to thoracoscopic esophagectomy in recent years owing to a worldwide trend of minimally invasive surgery (MIS).
Respiratory care protocol
Respiratory care was administered to patients receiving esophageal surgery by a respiratory therapist throughout the duration of hospitalization both in group A and B. Pre-operative assessment included incentive spirometry, abdominal breathing, and appropriate cough technique. Post-operative assessment included the above items, as well as optimization of oxygen delivery, inhalation medical therapy, and the process of weaning from mechanical ventilation if necessary.
About anesthesia
Pulmonary aspiration is a risk and should be minimized as much as possible with H2 blocker or proton-pump inhibitor preoperatively. Awake nasogastric suctioning may also help.
Double-lumen endobronchial tube (Mallinckrodt Inc., St. Louis, MO, USA) was routine for both conventional and VATS esophagectomy.
Thoracic epidural analgesia was administered, as routine, for post-operative pain control.
Statistical methods
Descriptive statistics were compiled and all data was expressed as mean ± standard deviation and number (percentage). Student t-test for continuous data and Chi-square with Fisher’s exact test was used for category data to determine the difference between the two time-groups, year 2008–2011 and year 2012–2016. Additionally, non-parametric Mann-Whitney U test was also used to determine the difference of time variables, such as age, ICU stay in days, days on ventilator, days of post-operative stay, and operative time between the two time-groups. Statistical analyses were performed using SPSS 20 for Windows. All statistical tests were two-tailed and P value of <0.05 was considered as statistically significant.
Results
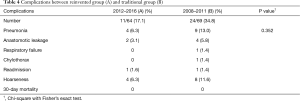
Demographic data is shown in Table 2, and short-term post-operative outcomes are shown in Table 3. Age (P=0.415), gender (P=0.682), pre-op CCRT (P=0.123) and ECOG (P=0.153) are determined to be of no statistical significance. The number of mid-third tumor in Group B was more than that of Group A. Group A showed significantly lower ventilator days (P<0.001), ICU stays (P<0.001), and post-operative stay (P<0.001)and more dissected three-field lymph nodes (P<0.001). Operative time, intra-operative blood loss and blood transfusions were statistically insignificant. There was no significant difference in complications rates between the two groups (P=0.300). Of them, pneumonia rate was reduced from 13.0% to 6.3% and anastomotic leakage rate from 5.8% to 3.1%. No hospital mortality was recorded (Table 4). The hospital charges in Group A were perceptively less than that of Group B (Table 3), although P value (P=0.078) did not reach statistical significance.
Full table
Discussion
According to the results of our study, the implementation of our new perioperative management protocol for esophagectomy and reconstruction was safe and feasible. Notably, post-operative complications and mortality had not increased. This can be explained in the following discussion.
In Group A, with the exception of one patient, all were extubated successfully in the operating room. The one patient who kept his tube to ICU was a 74-year-old male with FEV1: 1.11 L. He was slowly weaned off the ventilator and extubated during a 4-day stay in ICU. Only two patients were re-intubated owing to ineffective cough and profuse sputum. They were extubated the next day without any difficulty. In the past, surgeons were wary of early extubation, owing to fear of re-intubation in the event of respiratory fatigue and pain after major operation. Lack of round-the-clock experienced staff was a concern. The current concept of prolonged intubation is a risk factor for pneumonia and systemic inflammatory reaction (5). Whereas, early extubation may shorten or even avert intensive care stay (6), as well as circumvent the use of ventilation.
It must be noted, however, that uneventful anesthesia is a prerequisite for early extubation and smooth recovery. We were able to achieve this by carefully considering the following two factors. First, we utilized a fixed anesthesiologist who was familiar with esophageal surgery, and integrated with our surgical team. Second, hemodynamic stability was maintained through optimal fluid management. Urinary output has formerly been the indicator of fluid adequacy, but new monitoring device such as stroke volume variation, if carefully used, bearing in mind that they are currently not without imperfection, could optimize perioperative perfusion, decrease incidence of post-operative lung edema, acute respiratory distress syndrome, and anastomotic leakage (7).
We routinely control post-operative pain with thoracic epidural analgesia. It has been shown to reduce the incidence of respiratory complications after surgery (5,8). Moreover, it improves graft microcirculation and prevents anastomotic insufficiency (9). A critical note is that the anesthesiologist should always stay at his or her post during the operation due to the unpredictable likelihood of arrhythmia and even hypotension that would occur during mediastinal dissection. Closed clear communication between the anesthesiologist and surgeon is essential. In some series, the ICU was bypassed, and the patients were transferred to the ward after close observation in post-anesthesia care unit (10). However, our philosophy was that all patients, stable or otherwise, although extubated, should be sent to ICU for immediate care. Despite the chances being low, we could not risk sudden aggravation. The total hospital stay would not be prolonged even if ICU stay was one or two days longer. The only disadvantage is higher cost.
All our patients started walking on the first postoperative day in Group A. As we know, early ambulation promotes circulation and improves peristalsis. It is advantageous for patients to return to enteral feeding as soon as possible (11) so that nutritions can be obtained alimentarily and side-effects of total parenteral nutrition lessened.
Bronchofiberscopic pulmonary toilet is a routine procedure on the first postoperative day in Group A. Most patients had the problem of effective cough, so pulmonary infection was a potential hazard. Compared with the literature (12-14), our pneumonia rate was much lower than expected.
It is worth mentioning that we encouraged families to come into the ICU and help patients to walk around while they were waiting to be transferred to ward throughout the group A and B. This not only decreased the load on nursing staff, but also allowed patients to reunite with their family much sooner.
Another factor contributing to the short hospital stay is our low anastomotic leakage rate.
Anastomotic leakage would potentially extend the hospital stay for more than a week. Due to the refinement of operative technique in reducing anastomotic leakage, as we had described previously (15), the rate of anastomotic leakage was only 3.1% compared with the rate reported in the literature of approximately 10–15% (12-14). Handling the stomach gently and preserving as many collaterals as possible is the key. We have to emphasize that we did all our cervical anastomose with hand-sewn technique, and the leakage rate was even lower than the reported rate by stapler method (16,17).
Postoperatively, we kept our patients NPO (nil per os) for six days instead of starting partake on the day after operation (18). Nutrition is through jejunostomy enterally.
First, we believe the healing of anastomosis will be more secure. Second, which was not mentioned in most of the literature, is that a certain number of patients would choke during first swallowing after the operation. This would cause aspiration if patients were too weak to cough up. That is why we let our patients drink several days later, giving them sufficient time to gradually recuperate, and greatly reducing the possibility of aspiration. With regard to the problem of NG tube removal, we decided on the delay after early removal prompted incidences of vomiting. The patients started drinking on the next day to confirm that they had no swallowing difficulty or choking. After discharge, if swallowing was smooth, they were encouraged to try soft diet. All patients were followed-up at outpatient clinic. Nutritional status of course gradually improved with soft diet and even common diet. One thing we have to mention is that there was only one re-admission in the new protocol group, and this was not related to swallowing. Our protocol appears more conservative than others found in the literature, but patient recovery is comparable, with lower than reported complication rate.
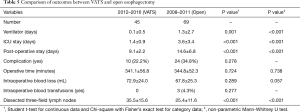
Respiratory complication (6.3%) was also low in our series, attributed to the help of respiratory therapists. One thing we should admit is that the implementation of VATS esophagectomy—a MIS—in the last four years maybe one of the reasons for this improvement. As we know, VATS may decrease the rate of pulmonary complication and length of stay (19-21). In our series, we cannot say whether our current results are affected by this, and further research is required in this area. In particular, we also made comparison between VATS in Group A and open esophagectomy in Group B (Table 5). Similar to Table 3, the VATS-based Group A showed comparable result with significantly lower ventilator days (P<0.001), ICU stays (P<0.001), and post-operative stay (P<0.001). Besides, the result yielded no statistic significance with regard to operative time, intra-operative blood loss, and intra-operative blood transfusions. But there were significantly more dissected three-field lymph nodes in Group A (P<0.001).
Full table
Moreover, according to the Table 2, patients in Group B seemed to be suffered from more advanced pathologic stage, less frequent use of CCRT and poorer ECOG, these cannot be precluded as factors causing better outcome of the Group A. But they are not statistically significant.
The hospital charges were conspicuously lower in Group A, although statistically insignificant. The difference was about US$1,600 between the groups. It seems small of course, but it is currently the average two-month salary for an university graduate in Taiwan. This cost reduction is an encouraging news, probably related to the significant reduction of ventilator use, and ICU and hospital stay. We also note that hospital charges in Taiwan are tremendously low when compared with that of U.S. (14).
The strong point of this series is that we were able to minimize bias by drawing on the patients of the same one surgeon, in this single particular institute. Not only we do obviate the technical discrepancies that inherently exists among surgeons, but the facilities and policies that vary between institutions have also been kept constant. The limitations of this study are (I) case number still small; (II) the higher proportion of MIS in the last three years that may shorten the recent hospital stay; (III) the improvement of dexterity and growing of experience may abridge the post-operative stay year by year; (IV) quality of life has not been compared because of the lack of this information from the patients of Group B.
In conclusion, our result showed that newly-adopted reinvented perioperative management protocol for esophagectomy and reconstruction for esophageal squamous cell carcinoma is feasible, safe, and cost-effective. With the implementation of this protocol, the ICU days and post-operative days were shortened significantly without increasing complication and mortality. Meanwhile, the hospital charges were also reduced concomitantly.
Acknowledgements
The authors thank the help of Jadzia and Andre Chou on language revision.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All data was obtained as part of routine clinical care from charts so that extra patient consent was waived. The study was approved by IRB [KMUH-IRB-E(II)-20150240].
References
- Health Promotion Administration, Ministry of Health and Welfare, Taiwan. Cancer Registry Annual Report 2013. Available online: http://health99.hpa.gov.tw/EducZone/edu_detail.aspx?CatId=21759, accessed October 9, 2016.
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. [Crossref] [PubMed]
- Watkins AC, White PF. Fast-tracking after ambulatory surgery. J Perianesth Nurs 2001;16:379-87. [Crossref] [PubMed]
- Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Lanuti M, de Delva PE, Maher A, et al. Feasibility and outcomes of an early extubation policy after esophagectomy. Ann Thorac Surg 2006;82:2037-41. [Crossref] [PubMed]
- Chandrashekar MV, Irving M, Wayman J, et al. Immediate extubation and epidural analgesia allow safe management in a high-dependency unit after two-stage oesophagectomy. Results of eight years of experience in a specialized upper gastrointestinal unit in a district general hospital. Br J Anaesth 2003;90:474-9. [Crossref] [PubMed]
- Thiele RH, Bartels K, Gan TJ. Inter-device differences in monitoring for goal-directed fluid therapy. Can J Anaesth 2015;62:169-81. [Crossref] [PubMed]
- Safranek PM, Cubitt J, Booth MI, et al. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg 2010;97:1845-53. [Crossref] [PubMed]
- Lázár G, Kaszaki J, Abrahám S, et al. Thoracic epidural anesthesia improves the gastric microcirculation during experimental gastric tube formation. Surgery 2003;134:799-805. [Crossref] [PubMed]
- Findlay JM, Tustian E, Millo J, et al. The effect of formalizing enhanced recovery after esophagectomy with a protocol. Dis Esophagus 2015;28:567-73. [Crossref] [PubMed]
- Hjort Jakobsen D, Sonne E, Basse L, et al. Convalescence after colonic resection with fast-track versus conventional care. Scand J Surg 2004;93:24-8. [Crossref] [PubMed]
- Markar SR, Karthikesalingam A, Low DE. Enhanced recovery pathways lead to an improvement in postoperative outcomes following esophagectomy: systematic review and pooled analysis. Dis Esophagus 2015;28:468-75. [Crossref] [PubMed]
- Blom RL, van Heijl M, Bemelman WA, et al. Initial experiences of an enhanced recovery protocol in esophageal surgery. World J Surg 2013;37:2372-8. [Crossref] [PubMed]
- Shewale JB, Correa AM, Baker CM, et al. Impact of a fast-track esophagectomy protocol on esophageal cancer patient outcomes and hospital charges. Ann Surg 2015;261:1114-23. [Crossref] [PubMed]
- Chou SH, Li HP, Lee JY, et al. Radical resection or chemoradiotherapy for cervical esophageal cancer? World J Surg 2010;34:1832-9. [Crossref] [PubMed]
- Liu QX, Min JX, Deng XF, et al. Is hand sewing comparable with stapling for anastomotic leakage after esophagectomy? A meta-analyisis. World J Gastroenterol 2014;20:17218-26. [Crossref] [PubMed]
- Okuyama M, Motoyama S, Suzuki H, et al. Hand-sewn cervical anastomosis versus stapled intrathoracic anastomosis after esophagectomy for middle or lower thoracic esophageal cancer: a prospective randomized controlled study. Surg Today 2007;37:947-52. [Crossref] [PubMed]
- Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al. Immediate postoperative oral nutrition following esophagectomy: a multicenter clinical trial. Ann Thorac Surg 2016;102:1141-8. [Crossref] [PubMed]
- Decker G, Coosemans W, De Leyn P, et al. Minimally invasive esophagectomy for cancer. Eur J Cardiothorac Surg 2009;35:13-20. [Crossref] [PubMed]
- Gemmill EH, McCulloch P. Systematic review of minimally invasive resection for gastro-oesophageal cancer. Br J Surg 2007;94:1461-7. [Crossref] [PubMed]
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]


