First generation bioresorbable vascular scaffolds: do they hold the promise?
The current generation of drug eluting stents (DES) exhibit a very low risk of stent thrombosis, restenosis, and need for repeat intervention (1). However, it took almost a decade for DES to reach this degree of maturation. Yet, these devices still carry a risk of very late stent restenosis and the need for repeat revascularization, due to the persistence of the metallic backbone and/or residual polymer once the anti-proliferative agent has completely eluted. As such, bioresorbable vascular scaffolds (BVS) hold the promise of offering an early anti-proliferative effect in addition to the mechanical support similar to DES for 2–3 years followed by complete bioabsorption. These effects are theoretically appealing. Complete bioabsorption could allow for preservation of the coronary vasomotion (which has been linked to the increased risks of late stent restenosis with DES), a possible reduction in the duration of dual antiplatelet therapy (DAPT) following the stent implantation, and the preservation of native coronary architecture which would allow for future surgical therapy.
The Absorb™ (Abbott Vascular, Abbott Park, IL, USA) BVS is the most commercially advanced among the different BVS in development, and the only BVS that is approved by the US Food and Drug Administration. In order to validate the widespread use of this device, it would be expected that BVS would be at least non-inferior to the available second generation DES between 2 and 3 years, and then show clear superiority afterwards. The available randomized trials, which have compared BVS with everolimus-eluting stents (EES), have been generally underpowered to determine any difference in hard clinical outcomes (such as stent/scaffold thrombosis, myocardial infarction, and death). Unfortunately, a meta-analysis of six randomized trials showed that the risk of stent/scaffold thrombosis might be higher as compared with EES at 1 year (2). More recently, these individual trials have reported disappointing outcomes at 2 years.
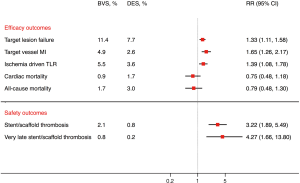
In this context, Sorrentino et al. performed an elegant meta-analysis of 7 randomized trials with 5,583 patients (3). At a median of 2 years, BVS was associated with an increased risk of target lesion failure, defined as cardiac death, myocardial infarction or target lesion revascularization [risk ratio (RR), 1.32; 95% confidence interval (CI), 1.10–1.59, number needed to harm (NNH) 41, P=0.003], as well as stent/scaffold thrombosis (RR, 3.15; 95% CI, 1.87–5.30, NNH 60, P<0.0001) as compared with EES. The risk of stent/scaffold thrombosis with BVS was concordant across the early (<30 days), late (30 days to 1 year), and very late (>1 year) periods (Pinteraction=0.49). These results were consistent with another meta-analysis of six randomized trials by Mahmoud et al. (4). At a mean of 25 months, BVS was associated with increased risk of target lesion failure, stent/scaffold thrombosis, very late stent/scaffold thrombosis (beyond 1 year), target vessel myocardial infarction, and target lesion revascularization compared with EES (Figure 1). These two meta-analyses add to the safety concerns previously noted with the use of BVS. Most concerning is the early increased risk of stent/scaffold thrombosis and also the very late (beyond 1 year) increased risk, which is reminiscent of first generation DES. Supporting this concern is the fact that real-world data demonstrate a higher than expected event rate with BVS at 2 years as well (5). These increased risks occur during a timeframe when BVS might be expected to begin to show superiority compared with EES. In lieu of these findings, the use of BVS in Europe and Australia has been restricted to clinical trials and registries. In addition, the US Food and Drug administration has recently issued a letter to physicians about the increased risk of major adverse cardiac events with Absorb™ BVS cautioning physicians to closely follow the instructions for target vessel selection (avoid in small vessels <2.25 mm).
Several hypotheses have been postulated to explain the increased risk of scaffold thrombosis with BVS. First, BVS has a significantly larger strut thickness (157 versus 81 µm with EES), which might translate into increased thrombogenicity as compared with EES. Second, some authors have expressed concern regarding the inadequate implantation technique which was adopted earlier on in BVS trials. A technique of pre-dilation, optimum sizing, and aggressive post-dilation (PSP technique) has been suggested in an attempt to reduce the risk of adverse events with BVS. The use of intravascular imaging, which has been shown to improve outcomes with DES (6,7), has been advocated to help ensure optimum sizing when implanting BVS. In an interim analysis of the ongoing ABSORB IV trial utilizing optimal high-pressure post-dilation (which has been achieved in ~86% of the patients who have been enrolled thus far), the risk of stent/scaffold thrombosis was lower than what was observed in these meta-analyses (0.4% at 30 days and 0.5% at 1-year for the pooled data for both EES and BVS) (8). This was not, however, reflected in the largest all-comers trial to date (the AIDA trial), in which pre-dilation and post-dilation were performed in most patients (98.6% and 74%, respectively) in the BVS group, yet the rate of scaffold thrombosis was ~3.5% (9). Third, the Absorb stent is composed of synthetic aliphatic polyesters [i.e., poly L-lactide (PLLA) and poly-D, L-lactide (PDLLA)] which are ultimately degraded into lactic acid. This could potentially lead to an inflammatory reaction and increased thrombogenicity. This is further supported by the fact that the absorbable magnesium scaffold backbone has shown promising results with acceptably low rates of thrombotic events. In one observational analysis, the risk of stent/scaffold thrombosis was 0% at 1-year with the absorbable magnesium scaffold backbone (10).
In summary, these two recent meta-analyses (3,4) add to the earlier concerns about the use of first generation BVS. It remains of utmost importance to adhere to optimal implantation techniques and to avoid BVS in smaller vessels. In those patients who already have a first generation BVS implanted, it might be reasonable to continue DAPT for at least 3 years. With longer procedure times, a more complex implantation technique, prolonged requirement for DAPT, and higher risk of ischemic or thrombotic events, the first generation of BVS do not yet hold the promise of improved outcomes. These findings should not, however, lead to an abandonment of interest in the potential of BVS. As with any first generation technology, future research should focus on defining the current pitfalls, improving the devices, and optimizing patient selection, which will hopefully lead to a realization of the promise of this technology.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Anderson is a consultant for BioSense Webster. Drs. Elgendy and Mahmoud have no conflicts of interest to declare.
References
- Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-term safety of drug-eluting and bare-metal stents: Evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2015;65:2496-507. [Crossref] [PubMed]
- Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet 2016;387:537-44. [Crossref] [PubMed]
- Sorrentino S, Giustino G, Mehran R, et al. Everolimus-eluting bioresorbable scaffolds versus everolimus-eluting metallic stents. J Am Coll Cardiol 2017;69:3055-66. [Crossref] [PubMed]
- Mahmoud AN, Barakat AF, Elgendy AY, et al. Long-term efficacy and safety of everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: A meta-analysis of randomized trials. Circ Cardiovasc Interv 2017;10:e005286. [Crossref] [PubMed]
- Wiebe J, Hoppmann P, Colleran R, et al. Long-term clinical outcomes of patients treated with everolimus-eluting bioresorbable stents in routine practice: 2-year results of the ISAR-ABSORB Registry. JACC Cardiovasc Interv 2017;10:1222-9. [Crossref] [PubMed]
- Elgendy IY, Mahmoud AN, Elgendy AY, et al. Outcomes with intravascular ultrasound-guided stent implantation: A meta-analysis of randomized trials in the era of drug-eluting stents. Circ Cardiovasc Interv 2016;9:e003700. [Crossref] [PubMed]
- Elgendy IY, Mahmoud AN, Elgendy AY, et al. Does the baseline coronary lesion length impact outcomes with IVUS-guided percutaneous coronary intervention? J Am Coll Cardiol 2016;68:569-70. [Crossref] [PubMed]
- Ellis SG. Everolimus-eluting bioresorbable vascular scaffolds in patients with coronary artery disease: ABSORB III Trial 2-Year results. American College of Cardiology Annual Scientific Session, Washington, DC, March 18, 2017.
- Wykrzykowska JJ, Kraak RP, Hofma SH, et al. Bioresorbable Scaffolds versus Metallic Stents in Routine PCI. N Engl J Med 2017;376:2319-28. [Crossref] [PubMed]
- Haude M, Ince H, Abizaid A, et al. Sustained safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de novo coronary lesions: 12-month clinical results and angiographic findings of the BIOSOLVE-II first-in-man trial. Eur Heart J 2016;37:2701-9. [Crossref] [PubMed]


