Ectopic right parietal pleural thymic carcinoma: a rare case and review of the literature
Introduction
Thymic neoplasms, such as thymoma and thymic carcinoma, arise mainly in the anterosuperior mediastinum, while ectopic thymomas account for only 4% of all thymomas (1). Thymic carcinoma is a rare tumor, representing less than 1% of thymic malignancies. Ectopic thymic carcinoma is extremely rare, and has only been reported in a handful of case reports in the English literature (2-5). Due to their unexpected location, ectopic thymic carcinomas are frequently misdiagnosed as one of the more common lesions seen at these sites. Although surgery is the most effective treatment for thymic carcinoma, it is difficult to excise in some cases due to tumor size, involvement of surrounding organs, or metastases. The diagnosis of ectopic thymic carcinoma is made by immunohistochemical evaluation. However, owing to the small number of cases that have been reported, optimal therapeutic strategies and prognostication are still lacking. Here, we report a case of ectopic thymic carcinoma that presented as a right parietal pleural tumor and was histologically diagnosed as thymic squamous carcinoma.
Case presentation
A 73-year-old man presented to our hospital for evaluation of a right intrathoracic tumor detected incidentally by computed tomography (CT) a year prior during his annual physical check-up. He was asymptomatic and exhibited no evidence of concomitant paraneoplastic syndrome. One year prior to presenting to the hospital, a chest CT demonstrated a sharp-edged soft tissue mass with mild enhancement measuring 31.4 mm × 8.9 mm with a broader base adjacent to the right axillary chest wall at the level of the 5th rib. There was no hilar or mediastinal lymphadenopathy or evidence of a mediastinal tumor or pleural nodules. There was no pleural effusion. The patient declined an operation and opted instead to observe the tumor over time and monitor for symptoms. After a year, a follow-up CT showed enlargement of the tumor (47 mm × 29 mm) with intense enhancement and a clear boundary adjacent to the ribs and intercostal tissue (Figure 1). An intrathoracic malignant tumor, most likely local malignant pleural mesothelioma or lung carcinoma with invasion into the parietal pleura, was suspected. CT images of the abdomen and skull showed no signs of lymph node enlargement or metastatic disease. An emission CT showed no signs of bone metastasis. Blood tests for tumor biomarkers were negative.
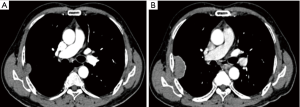
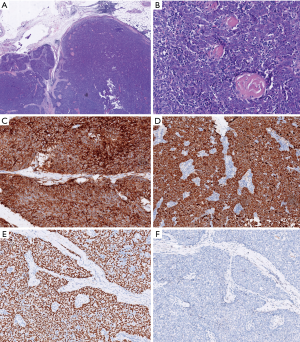
The patient was scheduled for surgery. He underwent a video-assisted thoracoscopic surgery. A 7 cm × 4 cm × 3 cm tumor was found with a pedicle stemming from the anterior parietal pleura. It was tightly adhered to the right upper lung. No pleural effusion or nodules were found. There was no connection between the tumor and the mediastinum or the thymus. There was no obvious thymus tissue around the tumor. A totally extra-pleural tumor resection with partial right lung resection was performed. Fast frozen tissue specimen showed a poor differentiated squamous cell carcinoma. Hilar and mediastinal lymph nodes were dissected. Histological examination demonstrated infiltrative growth typical of carcinoma cells with a nest-like distribution that locally invaded the surface of the lung tissue but did not involve the subpleural tissue of the parietal pleura. The carcinoma cells were large and oval with a prominent nucleolus. A small amount of lymphocytes had also infiltrated among the carcinoma cells. Hyperplasia of collagen fibers existed between the cell nests. Immunohistochemical examination was positive for CD5, P63, and CK5/6, but negative for TIF-1, CD56, synaptophysin (Syn), mesothelial cell (MC), calretinin (CR), Wilm’s tumor 1 protein (WT1), S-100, HMB-45, and Epstein-Barr virus RNA (EBER) (Figure 2). No lymph node metastases were found. These findings support a diagnosis of ectopic thymic squamous cell carcinoma. Cells that are CD5+ are specific for the diagnosis of primary thymic carcinoma (6). A final diagnosis of right parietal pleural ectopic thymic carcinoma was made.
The patient was discharged on the 5th postoperative day after an uneventful hospital course. Mediastinal radical radiotherapy (60 Gy) was performed postoperatively. The patient declined combined synchronous chemotherapy and thymectomy. After 17 months follow-up, the patient remained alive without any evidence of disease recurrence or metastasis.
Discussion
Thymic carcinomas are rare tumors (7) that usually occur in the anterior mediastinum. Rarely, however, ectopic thymic carcinoma can occur where the cancer is located outside of the mediastinum. Only 4 cases have been previously reported in the English literature. One case was a 49-year-old man who presented with cervical ectopic thymic squamous cell carcinoma with a microcarcinoma in his remnant mediastinal thymus (2). Another case was a 73-year-old man with an intrapericardial ectopic thymic carcinoma (3). A third case was a 24-year-old woman with cervical ectopic thymic squamous carcinoma (4). The fourth case was an 83-year-old man with ectopic intrathoracic undifferentiated thymic carcinoma (5).
Ectopic thymic neoplasms are thought to originate from aberrant thymic tissue that was displaced during embryologic development of the thymic gland (6,8-10). However, the histogenesis of ectopic thymic neoplasms remains a subject of speculation. While this theory, called the displacement theory, may explain the existence of thymic tissue in locations along the path of the developing thymus, it fails to explain the presence of thymic neoplasms in the lung and on pleural surfaces. The pulmonary system develops much earlier than the thymus (11). Primary intrapulmonary tumors deriving from ectopic tissues not native to the lung, such as meningiomas (12) or melanoma (13), support the hypothesis that ectopic thymic neoplasms may be able to originate from stem cells. These uncommitted germinative cells have the capacity to develop along many different cell lineages (14). While some other explanations have also been theorized (15), the displacement and stem cell theories remain the most widely accepted. The highest incidences of ectopic thymomas are in the cervical region followed by the lungs and pleura (1).
Though the cytologic features of ectopic thymomas and thymic carcinoma are identical to those of mediastinal thymomas and thymic carcinoma, the correct diagnosis is extremely challenging to make because of the rarity of being found in such an unusual location and the variety of histology patterns seen. Some pathologists lack of awareness of this entity and lack of typical features of the thymoma or thymic carcinoma make the diagnosis even more difficult. Moreover, a predominantly epithelial thymoma may be misdiagnosed as thymic carcinoma. In our patient, a fast frozen tissue specimen showed a typical poorly-differentiated squamous carcinoma. Due to the tumor site and histology pattern, the differential diagnosis should include: (I) malignant mesothelioma; (II) primary lung cancer, such as lung squamous cell carcinoma or lung lymphoepithelioma-like carcinoma invading the pleura; (III) metastatic carcinoma; (IV) ectopic thymic carcinoma; and (V) other primary pleural tumor, like melanoma. Positivity for P63 and CK5/6 indicated the tumor had epithelial characteristics. Negativity for Syn and CD56 indicated a lack of neuroendocrine features. Although the tumor tightly adhered to the right upper lung, primary lung cancer was excluded based on microscopic findings and negative expression of TTF-1 and EBER. Negativity for MC, CR, or WT1 suggested that malignant mesothelioma could be ruled out. However, immunohistochemistry cannot distinguish metastatic carcinoma from primary carcinoma without other radiological features. Melanoma was excluded due to the negative expression of S-100 and HMB-45, both specific for melanoma. CD5 positivity gave strong evidence for the diagnosis of thymic carcinoma according to consensus by WHO classification (16) and the International Thymic Malignancy Interest Group (17). Previous reports have depended on the expression of CD5 to diagnose ectopic thymic (2,4,5), as well as CD117 in the case of intrapericardial ectopic thymic carcinoma (3). Moreover, CD5, CD117, MUC1, and GLUT1 can be used as specific markers to distinguish thymoma from thymic carcinoma. However, a diagnosis of B3 thymoma should not be changed to thymic carcinoma in an otherwise typical B3 thymoma despite the expression of CD5, CD117, MUC1, or GLUT1 (18). Additionally, many nonthymic cancers express CD5, CD117, GLUT1, and MUC1. After combined immunohistochemistry analysis and exclusion of nonthymic cancer, the final diagnosis of right parietal pleural ectopic thymic carcinoma can be reached.
Postoperative therapy is limited and not standardized due to the low incidence of this disease. Complete resection, followed by chemotherapy and radiotherapy, is the generally-accepted strategy (19). After R0 resection of ectopic thymic carcinoma, Yao et al. suggested cisplatin-based chemotherapy and mediastinal radiotherapy in an ectopic neck thymic carcinoma (4). Hsu et al. suggested further thymectomy followed by chemotherapy and mediastinal and neck radiotherapy in another patient with ectopic neck thymic carcinoma (2). The patient in the literature with ectopic intrathoracic undifferentiated thymic carcinoma did not receive adjuvant therapy because of advanced age (5); further thymectomy revealed that there was a 0.15 cm thymic carcinoma in the remnant of the mediastinal thymus in addition to the larger 4.6 cm × 4.2 cm tumor in the neck. Thymectomy may be a reasonable strategy to clarify whether the tumor is ectopic or metastatic. It is difficult to distinguish between primary ectopic carcinoma and metastatic carcinoma if the primary carcinoma cannot be located. In our patient, postoperative radical mediastinal radiotherapy was performed without chemotherapy. There was no sign of disease recurrence 17 months after the operation.
Thymic carcinoma is an aggressive tumor. The 5-year survival rate for all patients is approximately 38–50%, with a significant survival time differential between low- and high-grade neoplasms (7). The 5-year overall survival was 0% in those with unresectable thymic carcinoma, with only an 11-month median survival (20), but was up to 82% in patients with complete resection (21). The improved survival owes to early diagnosis and multimodal treatment. After complete resection of ectopic tumors, the patients have been reported to survive without evidence of recurrence through follow-up ranging from 6 to 24 months (2,4,5), (17 months in our case). Complete surgical resection is the most definitive prognostic indicator for ectopic thymic carcinoma. Surgery should be attempted in all patients that are deemed operable irrespective of tumor site.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Weissferdt A, Moran CA. The spectrum of ectopic thymomas. Virchows Arch 2016;469:245-54. [Crossref] [PubMed]
- Hsu IL, Wu MH, Lai WW, et al. Cervical ectopic thymoma. J Thorac Cardiovasc Surg 2007;133:1658-9. [Crossref] [PubMed]
- Calderon AM, Merchan JA, Rozo JC, et al. Intrapericardial primary thymic carcinoma in a 73-year-old man. Tex Heart Inst J 2008;35:458-61. [PubMed]
- Yao WT, Chen CH, Lee JJ, et al. Ectopic thymic carcinoma in the neck. Ann Thorac Surg 2010;90:666-8. [Crossref] [PubMed]
- Matsuoka K, Murata Y, Ueda M, et al. Ectopic thymic carcinoma presenting as an intrathoracic mass. Asian Cardiovasc Thorac Ann 2016;24:480-3. [Crossref] [PubMed]
- Cordier AC, Haumont SM. Development of thymus, parathyroids, and ultimo-branchial bodies in NMRI and nude mice. Am J Anat 1980;157:227-63. [Crossref] [PubMed]
- Eng TY, Fuller CD, Jagirdar J, et al. Thymic carcinoma: state of the art review. Int J Radiat Oncol Biol Phys 2004;59:654-64. [Crossref] [PubMed]
- Gilmour J. Some developmental abnormalities of the thymus and parathyroids. J Pathol Bacteriol 1941;52:213-8. [Crossref]
- Loney DA, Bauman NM. Ectopic cervical thymic masses in infants: a case report and review of the literature. Int J Pediatr Otorhinolaryngol 1998;43:77-84. [Crossref] [PubMed]
- Norris EH. The morphogenesis and histogenesis of the thymus gland in man: in which the origin of the Hassall’s corpuscles of the human thymus is discovered. Contrib Embryol 1938;27:193.
- Moran CA, Suster S, Fishback NF. Primary intrapulmonary thymoma. A clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol 1995;19:304-12. [Crossref] [PubMed]
- Allen MS Jr, Drash EC. Primary melanoma of the lung. Cancer 1968;21:154-9. [Crossref] [PubMed]
- Drlicek M, Grisold W. Lorber. Pulmonary meningioma. Immunohistochemical and ultrastructural features. Am J Surg Pathol 1991;15:455-9. [Crossref] [PubMed]
- Marchevsky AM. Lung tumors derived from ectopic tissues. Semin Diagn Pathol 1995;12:172-84. [PubMed]
- Fukayama M, Maeda Y, Funata N. Pulmonary and pleural thymoma. Diagnostic application of lymphocyte markers to the thymoma of unusual site. Am J Clin Pathol 1988;89:617-21. [Crossref] [PubMed]
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014;9:596-611. [Crossref] [PubMed]
- Wu J, Fang W, Chen G. The enlightenments from ITMIG Consensus on WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Dis 2016;8:738-43. [Crossref] [PubMed]
- Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg 2015;149:95-100. [Crossref] [PubMed]
- Nakamura Y, Kunitoh H, Kubota K, et al. Platinum-based chemotherapy with or without thoracic radiation therapy in patients with unresectable thymic carcinoma. Jpn J Clin Oncol 2000;30:385-8. [Crossref] [PubMed]
- Hsu HC, Huang EY, Wang CJ, et al. Postoperative radiotherapy in thymic carcinoma: Treatment results and prognostic factors. Int J Radiat Oncol Biol Phys 2002;52:801-5. [Crossref] [PubMed]


