Patient reported outcomes following video assisted thoracoscopic (VATS) resection or stereotactic ablative body radiotherapy (SABR) for treatment of non-small cell lung cancer: protocol for an observational pilot study (LiLAC)
Introduction
Lung cancer is increasingly a disease of less fit patients with a median age of 70 years at diagnosis, with 1/3 aged 75 years or older (1). The diagnosis of stage I non-small cell lung cancer (NSCLC) is usually made in the absence of tumour-related symptoms. Poorer baseline performance status and health-related quality of life (HRQOL) are in the most part caused by comorbidities such as chronic obstructive pulmonary disease (COPD) (2). As a consequence, preservation of baseline HRQOL can be regarded as an optimal result of treatment for NSCLC. Furthermore, lung cancer has the highest mortality of all cancers in the UK, and accounts for the largest single cause of premature death in Leeds (3). The latest analysis of lung cancer incidence rates reports significant variation across the UK, with the highest rates in the north of England (4).
Currently there is insufficient data to identify clear-cut criteria for defining high-risk patients and help the clinicians and patients to make a decision about the best treatment for early stages of NSCLC. Furthermore, this data does not include patient reported outcome measures (PROMs) and does not account for the individual patients’ preferences. There is a clear need for supporting decision-making process using PROMs as a key part of information provision to patients.
PROMs are standardised and validated instruments to measure patients’ perceptions of their health status and their HRQOL (5).
The FDA definition of patient reported outcomes (PRO) is “any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else”. PROMs can include standardised and validated instruments to measure patients’ perceptions of their health status (6). PROMs can be used to assess patients’ HRQOL. HRQOL is considered to be a multidimensional functional effect of a medical condition and/or its consequent therapy upon a patient (7).
An existing NHS PROMs programme covers four common elective surgical procedures since 2009 (8). Despite evidence of correlations between PROMs and survival [including previous research from our group (9)], collection of this data after NSCLC surgery is still not routine.
Compared to the standard open lobectomy procedure, video-assisted thoracoscopic surgery (VATS) is a minimally invasive procedure with lower complication rates and shorter hospital stay compared to the traditional approach (10,11). The benefits of VATS are particularly evident in high-risk patients (12). VATS has gained increasing popularity over the last few decades to diagnose and treat lung cancer. In higher risk patients, a more limited anatomical sub-lobar resection or wedge excision may be performed (13,14). Although it has been said that the period of recovery post operation is shorter after undergoing a VATS, little is known about how VATS patients fare in terms of their QOL compared to patients who have undergone traditional open resections (15-17). As such, more studies need to be undertaken to explore the post-operative impact of VATS and open lung resections on patients’ daily lives.
Patients with potentially curable NSCLC, who may be at high operative risk due to co-morbidity, have a potential for achieving disease control and survival comparable to surgery with the stereotactic ablative body radiotherapy (SABR) (18-20). This approach has been developed thanks to major advances in technology, allowing much higher doses safely and accurately delivered over a significantly shorter treatment time and lower toxicity. SABR is an outpatient treatment with 3–8 treatments given according to tumour location using nationally agreed guidelines (21).
Only few long-term follow-up data are available for this treatment in terms of PROMs (22): however, there is no clinically meaningful decrease in HRQOL in patients with early-stage NSCLC during the first year after SABR (23).
The majority of UK patients want to be involved in decisions regarding their health status (24). Meaningful and sensitive patient-doctor communication around treatment options is a priority for NHS: the biggest predictor of legal complaint is not bad outcomes, but a combination of poor outcomes with bad communication (25). Data about the perceived involvement of the patient in the decision-making process or presence of any residual decisional conflicts may help to identify groups of patients requiring decision support. In this case it may be useful to include decision aids as part of process of care. Data on PROMs after treatment may be utilized in specific decision aids either paper-based (leaflets in clinics) or computer/Internet-based. Nevertheless, research findings indicate a poor rate of patient involvement during surgical consultations (26).
Further, patients who have been diagnosed with lung cancer may be unsure of which of these procedures to select. Factors to consider include: intraoperative and post-operative complications, length of hospital stay, post-operative recovery period and residual lung function. Undertaking a study to explore the impact of different treatment options on post-operative HRQOL may help patients in their decision-making process and to ultimately cope better with post-operative complications. This study would also help health care professionals understand the impact of different treatments on their patients QOL and offer new post-operative rehabilitation interventions to improve patient care. How we can improve the HRQOL for patients with cancer both during and after treatment has been largely investigated (27,28). However, it remains unclear whether information about HRQOL, adverse events (AEs) and patient satisfaction after different treatments may affect patient choice after the diagnosis of cancer.
Aim
This study aims to describe the trajectory of lung cancer patients HRQOL, symptoms and functions following VATS or SABR treatment for stage I–II NSCLC and to determine the feasibility and patient acceptability of online self-reporting of PROMs.
Specific objectives of the study include:
- To compare changes before and after treatment of PROs (HRQOL and patient satisfaction) after VATS lung resections or SABR in early stage lung cancer patients.
- To correlate clinical outcomes (complications and AEs) with quality of life in order to find objective predictors of major decline in PROs.
- To identify specific factors, which have influenced the personal choice between the treatments (decision self-efficacy scale).
- To establish recruitment and attrition rates and adherence to PROMs reporting during the study.
- To describe patient choice of electronic vs. paper questionnaires.
- To explore implementation issues through patient and staff interview.
Methods & analysis
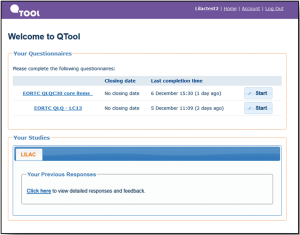
The proposed research is a prospective, observational study in cohort of 300 consecutive patients undergoing treatments for early stage NSCLC in Leeds Teaching Hospital Trust. A prospective study involves a group of similar individuals (in this case, patients treated with surgery and radiotherapy) and following them up over time (longitudinal). This will establish how different factors affect a certain outcome (in this case, HRQOL) and how that outcome may change over time. Patients will be invited to self-report symptoms, functions and HRQOL using the online secure QTool software at home or clinic (Figure 1). Paper administration will be offered to patients without Internet access.
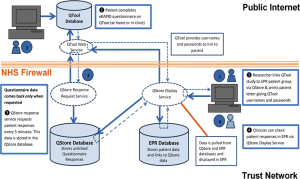
QTool is a web-based questionnaire collection system, developed and used as part of the NIHR funded development programme eRAPID (electronic patient self-Reporting of Adverse events: Patient Information and aDvice) (see Figure 2 for an overview) (29-31). This allows patients to self-report symptoms during and after treatment at home or clinic and has been integrated with patient pathway manager (PPM), Leeds and Yorkshire Cancer Network’s electronic patient records (EPRs) system. Assessments are analysed and viewed by clinicians for use in clinic. All data is collected in protected databases and can be analysed for the purposes of audit or research. QTool and PPM will be used in this project as the platform to create and analyse the electronic PROM questionnaire.
Patient demographic and clinical information will be collected prospectively (age, gender, co-morbidity, current medications, smoking status and treatment-specific information).
This study will use several outcomes to depict the trajectory of two lung cancer treatments from the patient point of view by using the following measures.
Patient-reported outcomes measures
We will assess overall quality of life and patient satisfaction using validated questionnaires [e.g., EORTC QLQ-C30 (32) with the LC-13 module (33) and Short-Form Patient Satisfaction Questionnaire PSQ-18 (34)]. We also explore the self-confidence or belief in one’s ability to make decisions, including participate in shared decision making with a validated instrument [decision self-efficacy scale (35-37)].
Clinical measures
We will collect pre- and post-treatments clinical information according the usual NHS care. In particular, we aim to collect:
- Baseline demographic and clinical information.
- Personal details and demographics including height, weight, and gender.
- Date of diagnosis.
- Pre-operative investigations results.
- Confirmation of eligibility.
- Confirmation of written informed consent.
- MDT decision: to be extracted by clinical letter on PPM.
- Internet access.
- Comorbidity, ECOG performance status, MRC dyspnoea score, Charlson co-morbidity index, pulmonary function tests, cardio-pulmonary exercise test (CPEX) and/or shuttle walk test (ISWT), NSCLC clinical stage.
Treatment and post-treatment clinical information
Surgical group
Surgeon, details of previous thoracic operations, whether the outcome was curative, palliative or unresectable in the opinion of the surgeon at the time of operation, American Society of Anesthesiologists’ (ASA) classification of physical health status, type of operation, duration of hospital stay, operative and postoperative complications, readmissions, peak-flow, carbon monoxide diffusing capacity (DLCO) in Leeds patients only, details of any local or distant recurrence, any details of adjuvant treatment and its AEs.
Complications have been defined according to the standardized definitions proposed by The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases task forces (13). Complications will be graded according to the thoracic morbidity and mortality classification system (38,39).
SABR group
Dose planned, dose delivered, dates of delivery, post-SABR complications, unexpected admissions, DLCO, details of any local or distant recurrence.
Toxicity will be evaluated using common terminology criteria for adverse events (CTCAE) version 4.0 (40). Data will be extracted from MOSAIQ (radiotherapy delivery system) or the EPR as necessary.
Patient and staff interviews
Semi-structured staff, patient and carer interviews will be conducted to explore experiences of taking part to this research project and any recommendations for improvement.
Patient population
Any patient with early stages of NSCLC (I–II) planned to have a VATS resection or a SABR treatment in Leeds Teaching Hospitals Trust who meet the inclusion and exclusion criteria are eligible (Table 1).

Full table
Informed consent
Participants will be recruited from Leeds Teaching Hospitals Trust.
Potential patients for this study will be identified by the clinical NSCLC teams during MDTs with the help of lung cancer clinical nurse specialists (CNS).
First approach and introduction to the study and consent: we felt that the best time to approach patients for the study is after the first outpatient clinic with surgeon or oncologist as at this stage there is a definite medical decision to proceed with active treatment and we can avoid the risk of approaching patients who subsequently don’t receive treatment.
After introduction from clinical staff, eligible patients will be approached by a member of the research team who will explain the study and provide the information sheet. Following information provision, patients will have as long as they need to consider participation (a minimum of 24 hours is advised) and will be given the opportunity to discuss the trial with their family and other healthcare professionals before they are asked whether they would be willing to participate.
If a patient would prefer more time to consider whether they wish to participate and will be given the opportunity to take the information home and discuss the study again with the researcher at their next visit (usually the booked pre-assessment for surgical patients and planning CT appointment for SABR patients). Patients have the opportunity to decline participation at this point, consent to participation at this point or to consider being involved and speak to the researcher at their next hospital visit.
If the patient is happy to participate (either at this first approach or a subsequent hospital visit) they will be asked to provide written informed consent. Patients who will prefer to fill the questionnaires electronically will receive training in using the QTool system. A member of the clinical trial team who received GCP training is permitted to take consent. A record of the consent process will be kept in patient’s notes. The patient will be free to withdraw from the study at any time without giving reasons.
Patient self-reported outcomes
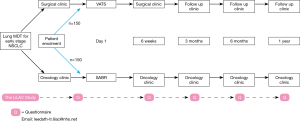
Outcome measures will be collected prior to treatment, and at 6 weeks, 3, 6 & 12 months afterwards, administering standard questionnaires via a remote web-based system (QTool). Paper administration will be offered to patients without Internet access. All patients will have a clinical report form (CRF) which would be in two different versions (surgery/SABR). The schedule for the study and the data collection is presented on Figure 3.
Data collection and management
Planned PROMs follow-up would be completed remotely online or by posting questionnaires to patients with a pre-paid addressed envelope. The questionnaires’ results will be provided to the clinician seeing the patient in the EPR. All clinicians will be trained in how to access to these results in EPR and how to interpret the scores.
Data management system
The electronic case report form (eCRF) system has been developed within the Patient Reported Outcomes Group (POG) at the Leeds Institute of Cancer and Pathology. The purpose of this software is to provide the research team with a means of accurately capturing clinical and anonymised patient related data for analysis in psychosocial oncology trials. All patient entry will be entered in the eDMS and linked in the electronic patients record to the QTool to match the questionnaires answers.
It will send out automatic reminders to patients to complete questionnaires on-line on an individual scheduled basis.
Interim and final interviews
Interim (at 6 months) and end-of-study interviews with patients and staff will give in-depth understanding of pros and cons of the project. Participants will be purposively sampled: patients by treatment, age below and over 60, gender; staff by role and specialty (surgery, oncology, respiratory medicine, nursing, and consultants vs. trainees). An end of study questionnaire will be sent electronically or by mail to all the patient participants along with the last HRQOL survey (at 12 months). We aim to recruit 5 patients and 5 healthcare providers for the interim interviews and 12 patients and 5 healthcare providers for the final interviews.
All the interviews will take place in private rooms within a clinical area at the hospital. These interviews will be audio recorded and will last for approximately 30 minutes.
Study outcomes
The following outcomes will be measured.
Primary outcomes
- HRQOL over time will be measured at baseline, 6 weeks, 3, 6 and 12 months.
- Patient satisfaction will be measured at 6 weeks.
- Self-confidence or belief in one’s ability to make decisions, including participate in shared decision making in the baseline assessment.
Secondary outcomes
- Correlation between clinical outcomes with HRQOL in order to find objective predictors of major decline in PROMs.
- Establish recruitment and attrition rates and adherence to PROMs reporting during the study.
- Patient preference in the mode of questionnaire administration (paper vs. electronic).
Descriptive outcomes
- Usefulness of the HRQOL information to physicians—interviews will be completed by doctors and health care providers at the end of the study.
- Patients’ attitudes and rating of usefulness of regular QOL measurement.
- Implementation issues around PROMs collection in standardize clinical practice.
Sample size
We determined the sample size of the surgical arm by using our historical cohort of 115 anatomic lung resections patients who completed the preoperative quality of life survey (operated in 2014). Their average baseline EORTC QLQ-C30 Global Health Scale value was 65 with a standard deviation of 21.5. Therefore, in order to detect a minimum peri-operative difference of 6.5 points (10% from baseline), with a two-sided alfa level of 0.05 and a statistical power of 90%, a sample size of 115 patients in the surgical arm was estimated. A similar assumption is made for SABR patients where we do not have any available data.
For longitudinal studies involving regular PROMs, we typically see 70% consent rate and 30–35% attrition over 3 months (41). Therefore, our expectations are to be able to recruit 150 VATS and 150 SABR patients over 12 months with 12 months of follow-up.
Proposed analysis
The feasibility of the recruitment strategy will be evaluated by summarising the screening, eligibility, and consent processes, including the number of participants involved at each stage. Where available, reasons for ineligibility and non-participation in the study will be summarised. Retention during follow-up, including the number of participants withdrawing from the study, the timing and reasons for withdrawal will be presented. Acceptability and potential conditions for improved acceptability will be explored as part of the patient and staff interviews.
The EORTC questionnaires scores over time will be summarised by treatment. Changes in score over time and differences between treatment arms will be explored using a multilevel repeated measures model.
Descriptive analysis of QOL, patient’s satisfaction and patient decision scale will be completed. The comparison between the two treatment arms will be performed using the Mann Whitney test for numeric variables and the Chi square test for categorical variables. A P value lower than 0.05 will be accepted as statistically significant.
Correlation between PROMs data and clinical outcomes will be explored by linear regression analysis adjusting by type of treatment and baseline characteristics of patients.
The interviews will be audio recorded; transcribed and detailed notes will be made alongside each item to guide analysis. A thematic content analysis will be applied to the data and emergent themes will be coded (42). Feedback from these will be used to make improvements for future implementations.
Strengths and limitations
This is the first project where RROMs are the primary end-point after early stage Non-small cell cancer patients’ treatment. The study will use a lung cancer specific questionnaire to follow up the patients for the entire year after surgery or radiotherapy treatment.
The use of electronic platform is an innovative way of reporting symptoms especially in NSCLC patients. We already audited the internet access of our patients in Leeds, and 60% of them declared to have it, at home or by their relatives and friends.
However, this is not a randomised trial. Although other studies comparing SABR to surgery in a randomized way have closed early due to a poor recruitment rate (10,43), we acknowledge the different type of patient groups. The LiLAC study is not aiming to draw conclusions form direct comparisons, but more to look at the trajectory of recovery post-treatments and preservation or improvement of the HRQOL.
Ethics & dissemination
This study has been approved by the NRES Yorkshire and the Humber-Leeds East Research Ethics Committee (REC Ref: 16/YH/0407). All substantial amendments will be submitted to the ethics committee for their approval prior to implementation.
Safety reporting
There will be no risks associated with participation in any aspect of the described study. However, in general, it is possible that some of the interview questions, and/or symptom and PROMs questions may cover sensitive issues or could uncover difficult emotions for patients. We will reiterate that participants can withdraw from the study at any time. We have an established procedure for dealing with patients who are distressed. If distress is detected, the researcher will inform a relevant member of the clinical team who are directly involved with their care, having obtained permission from the patient first.
Trial monitoring and oversight
To ensure arrangements and systems are in place for the management and monitoring of the study, we have set up several, regular meetings to aid and review the research.
The research team meet once a week to discuss and monitor all trials that the group are involved in and this study is covered in that meeting. The LILAC research team also hold regular weekly/monthly (as appropriate) management workgroup meetings to discuss and monitor the progress of the study. Advisors to the team (including clinical and patient representatives, trial statistician) will be part of the specific study management group (SMG), and updates to these individuals will be provided at least 3 months via email, teleconference or meetings in person if necessary.
Every 6 months the project steering committee will meet in person or by phone to monitor the study.
If there are arising safety/ethical issues, these can be raised with the steering committee via email.
Dissemination
The results of this study will be submitted for publication in international peer-reviewed journals. Additionally, key results will be presented at relevant national and international conferences. Finally, the results of this study will part of the first author’s PhD thesis.
Acknowledgements
The authors would like to acknowledge the support of all the PCOR group.
Funding: This work was supported by Yorkshire Cancer Research, grant number L399.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the NRES Yorkshire and the Humber-Leeds East Research Ethics Committee (No. 16/YH/0407).
References
- Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer 2002;94:2766-92. [Crossref] [PubMed]
- Pompili C, Brunelli A, Refai M, et al. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg 2010;37:525-30. [Crossref] [PubMed]
- Cancer in Yorkshire. Statistics. Available online: http://yorkshirecancerresearch.org.uk/cancerinyorkshire/
- UK CR. Cancer Statistics Key Facts - All cancers combined. 2014. Available online: http://www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/Allcancerscombined/
- Greenhalgh J, Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract 1999;5:401-16. [Crossref] [PubMed]
- Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 2006;4:79. [Crossref] [PubMed]
- What Is Health-Related Quality of Life Research? Available online: http://www.isoqol.org/about-isoqol/what-is-health-related-quality-of-life-research
- Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013;346:f167. [Crossref] [PubMed]
- Pompili C, Salati M, Refai M, et al. Preoperative quality of life predicts survival following pulmonary resection in stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2013;43:905-10. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 16-8.
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [Crossref] [PubMed]
- Li WW, Lee TW, Lam SS, et al. Quality of life following lung cancer resection: video-assisted thoracic surgery vs thoracotomy. Chest 2002;122:584-9. [Crossref] [PubMed]
- Rizk NP, Ghanie A, Hsu M, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg 2014;98:1160-6. [Crossref] [PubMed]
- Franks KN, Jain P, Snee MP. Stereotactic ablative body radiotherapy for lung cancer. Clin Oncol (R Coll Radiol) 2015;27:280-9. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Jain P, Baker A, Distefano G, et al. Stereotactic ablative radiotherapy in the UK: current status and developments. Br J Radiol 2013;86:20130331. [Crossref] [PubMed]
- Chen H, Louie AV, Boldt RG, et al. Quality of Life After Stereotactic Ablative Radiotherapy for Early-Stage Lung Cancer: A Systematic Review. Clin Lung Cancer 2016;17:e141-9. [Crossref] [PubMed]
- Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol 2012;7:1148-54. [Crossref] [PubMed]
- Magee M. Relationship-Based Health Care in the United States, United Kingdom, Canada, Germany, South Africa, and Japan. A Comparative Study of Patient and Physician Perceptions Worldwide. 2003.
- Tamblyn R, Abrahamowicz M, Dauphinee D, et al. Physician scores on a national clinical skills examination as predictors of complaints to medical regulatory authorities. JAMA 2007;298:993-1001. [Crossref] [PubMed]
- Hawley ST, Lantz PM, Janz NK, et al. Factors Associated with Patient Involvement in Surgical Treatment Decision Making for Breast Cancer. Patient Educ Couns 2007;65:387-95. [Crossref] [PubMed]
- Raz DJ, Sun V, Kim JY, et al. Long-Term Effect of an Interdisciplinary Supportive Care Intervention for Lung Cancer Survivors Following Surgery. Ann Thorac Surg 2016;101:495-502; discussion 502-3. [Crossref]
- Clark MM, Novotny PJ, Patten CA, et al. Motivational readiness for physical activity and quality of life in long-term lung cancer survivors. Lung cancer 2008;61:117-22. [Crossref] [PubMed]
- Ashley L, Jones H, Thomas J, et al. Integrating cancer survivors' experiences into UK cancer registries: design and development of the ePOCS system (electronic Patient-reported Outcomes from Cancer Survivors). Br J Cancer 2011;105 Suppl 1:S74-81. [Crossref] [PubMed]
- Holch P, Warrington L, Potrata B, et al. Asking the right questions to get the right answers: using cognitive interviews to review the acceptability, comprehension and clinical meaningfulness of patient self-report adverse event items in oncology patients. Acta Oncol 2016;55:1220-6. [Crossref] [PubMed]
- Warrington L, Absolom K, Velikova G. Integrated care pathways for cancer survivors - a role for patient-reported outcome measures and health informatics. Acta Oncol 2015;54:600-8. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Koller M, Warncke S, Hjermstad MJ, et al. Use of the lung cancer-specific Quality of Life Questionnaire EORTC QLQ-LC13 in clinical trials: A systematic review of the literature 20 years after its development. Cancer 2015;121:4300-23. [Crossref] [PubMed]
- Marshall GNaRDH. The Patient Satisfaction Questionnaire Short Form (PSQ-18). 1994.
- O'Connor AM. User Manual – Decision Self-Efficacy Scale. Ottawa: Ottawa Hospital Research Institute, 1995.
- Bunn H, O'Connor A. Validation of client decision-making instruments in the context of psychiatry. Can J Nurs Res 1996;28:13-27. [PubMed]
- Cranney A, O'Connor AM, Jacobsen MJ, et al. Development and pilot testing of a decision aid for postmenopausal women with osteoporosis. Patient Educ Couns 2002;47:245-55. [Crossref] [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 42. [Crossref] [PubMed]
- Ivanovic J, Al-Hussaini A, Al-Shehab D, et al. Evaluating the reliability and reproducibility of the Ottawa Thoracic Morbidity and Mortality classification system. Ann Thorac Surg 2011;91:387-93. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events version 4.0.
- Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol 2004;22:714-24. [Crossref] [PubMed]
- Ritchie J, Spencer L. Qualitative data analysis for applied research. Analysing qualitative data, 1994.
- Murray P, Franks K, Hanna GG. A systematic review of outcomes following stereotactic ablative radiotherapy in the treatment of early-stage primary lung cancer. Br J Radiol 2017;90:20160732. [Crossref] [PubMed]