Analysis of the clinical differentiation of pulmonary sclerosing pneumocytoma and lung cancer
Introduction
Pulmonary sclerosing pneumocytoma (PSP) is a rare benign lung tumor (1,2), which was previously known as pulmonary sclerosing hemangioma (PSH). It was reported and named in 1956 by Liebow (3) and renamed as PSP in 2015 by the World Health Organization (4). Diagnostic confirmation of PSP relies on surgical pathology, as its preoperative imaging performance lacks specificity; therefore, the differential diagnosis of PSP and lung cancer is especially difficult. This study focused on PSP patients and investigated imaging and other comprehensive clinical characteristics of PSP cases compared with lung cancer cases, thus accumulating the diagnostic experience for such patients.
Methods
Patient characteristics
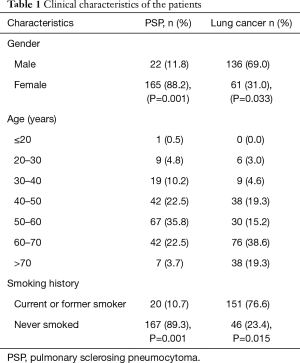
This study was a retrospective study, collected data on PSP patients who were treated at the Shanghai Pulmonary Hospital, Tongji University School of Medicine from December 2012 to February 2017. This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine (ethics approval No. K17-137). All patients had signed an informed consent. A diagnosis of PSP was confirmed in all patients via surgical resection. This study also collected data on lung cancer patients who were treated during the same period and whose diagnosis was confirmed via lung puncture cytology or surgical resection pathology. In all, 187 patients with PSP were enrolled in this study, and 197 patients with lung cancer were enrolled (Table 1).
Full table
Enrollment criteria
We screened for new patients who had not received a conformation of diagnosis and who had not received treatment. Prior to the diagnosis, patients underwent enhanced computed tomography (CT) imaging [Siemens SOMATOM (Siemens Medical Solutions, Germany)]. CT images revealed solid nodules or clumps in the lung, and clinical imaging indicated the possibility of benign lung tumor or lung cancer. The patients were hospitalized in our hospital and were diagnosed with either PSP or lung cancer.
Pathological diagnosis method
For all enrolled PSP patients, lesion samples were obtained after surgery in our hospital and were subjected to pathological examination to confirm the diagnosis. Lung cancer patients underwent CT-guided percutaneous lung puncture cytology or pathological examination after surgical resection for confirmation of the diagnosis.
Observation indicators
For patients who were pathologically diagnosed with PSP or lung cancer, we observed their size, location, and morphology of the lung lesions, and for patients who received surgical treatment, we also observed the presence or absence of a capsule, vascular involvement, the presence or absence of pleural adhesions, and whether surgical complications occurred.
Statistical analysis
Statistical analysis was performed using the Statistical Software Package (SPSS for Windows, version 21.0; SPSS Inc., USA). Two groups of data were compared using the t-test, and P<0.05 was considered to be statistically significant.
Results
Patient characteristics
For enrolled PSP patients, the mean age was 52.3±6.7 years, and the ratio of females (n=165, 88.2%) to males (n=22, 11.8%) was 7.5:1. This disease was often found in the 40–70-year age group, where it accounted for 80.8% of the total number of cases. The most frequently affected age group was the 50–60-year age group, in which 67 cases (35.8%) were included. One patient was only 15 years of age, whereas 7 patients were older than 70 years of age (3.7%). The average age of the patients with lung cancer was 59.7±5.1 years of age, and the ratio of females (n=61, 31.0%) to males (n=136, 69.0%) was 0.45:1. Lung cancer was often found in those over 40 years of age, where it accounted for 92.4% of the total number of cases. The most frequently affected age group was the 60–70-year age group (76 cases, 38.6%). Eight PSP cases (4.3%) received thoracotomy, and 179 PSP cases (95.7%) received thoracoscopic surgery, of which 114 cases (61.0%) were treated with segmentectomy, and 73 cases (39.0%) were treated with lobectomy. In all, 118 lung cancer cases (59.9%) were confirmed by CT-guided percutaneous lung puncture cytology, and 79 cases (40.1%) were diagnosed by surgical resection followed by pathological examination.
Clinical manifestations
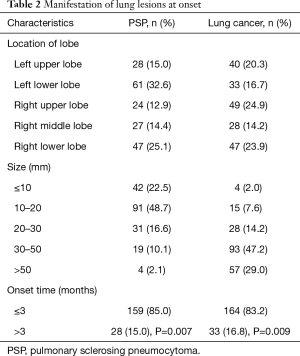
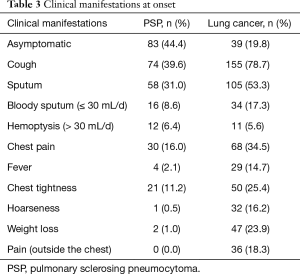
PSP and lung cancer patients might have lesions in all lobes of the lung (Table 2). According to this study, PSP often presented as lesions in the lower lobes, with lesions in the left lower lobe [61 cases (32.6%)] and the right lower lobe [47 cases (25.1%)], which accounted for 57.7% of the total number of cases. Lung cancer patients had lesions scattered in all lobes of the lung, but no obvious frequently affected areas were found. PSP lesions were relatively small, as the majority lesions were in the range of ≤20 mm, and most lesions were in the range of 10–20 mm [91 cases (48.7%)]. Lung cancer patients had larger lesions, as lesions >30 mm accounted for 76.2% of the total number of cases. Most PSP and lung cancer patients were newly identified [85.0%, and 83.2% (P=0.007; P=0.009)]. After disease onset, 44.4% of PSP patients exhibited no clinical manifestations (Table 3), and symptomatic patients with PSP or lung cancer often presented with cough and sputum. Other symptoms of PSP were not specific, and although 34.5% of patients with lung cancer experienced chest pain, this symptom was rare in PSP patients (16.0%).
Full table
Full table
CT findings before diagnosis
There were more PSP patients with smooth (with no short burrs) lesion boundaries than there were patients with rough (with short burrs) boundaries according to CT imaging (Table 4) (P=0.085). Significantly more lung cancer patients had lesions with unsmooth boundaries or short burrs in the boundaries (176 cases), which accounted for 89.3% of the total number of cases (P=0.001). Moreover, 82.4% of the PSP cases demonstrated no enlargement of the mediastinal lymph nodes, while most of the lung cancer patients had enlarged mediastinal lymph nodes (lymph nodes >10 mm in shortest diameter) (85.3%) (P=0.001). Most patients with PSP showed no calcification within the lesions (75.9%) or vascular involvement (79.1%), had hypodense shadow [with low Hounsfield units (≤30 units)] compared with muscle (62.0%), and had single lesions (97.9%), while lung cancer patients showed no calcification (81.2%) and had high CT values (67.5%); in addition, most lesions of lung cancer patients were associated with blood vessels (vascular involvement) (64.5%), and the internal texture (the CT values in tumor tissue) was not uniform (75.1%).

Full table
Postoperative follow-up
All enrolled PSP patients received a confirmation of diagnosis after pathological examination of surgically resected tissues. The postoperative results showed that 30 cases (16.0%) contained capsule or capsule-like structures surrounding the lesions, which were easily resected during surgery. Four cases were diagnosed as multiple PSP, of which 2 cases had multiple lesions in the same lobe of the lung, and 2 cases had lesions located in different lobes (1 case in the left upper lobe + left lower lobe, and 1 case in the right middle lobe + left lower lobe). Postoperative pathology revealed 1 case with ossification, 1 case associated with lymphangioma, and 1 case with interstitial vitreous changes. Of the 187 PSP patients who underwent surgery, 6 experienced postoperative bleeding (>500 mL), and 3 experienced pneumonia; all these patients improved after treatment. However, 1 case of pneumothorax, 1 case of chest pain, and 1 case of hoarseness were also observed, and although these were not life-threatening, these patients did not improve after treatment. None of the patients with PSP experienced recurrence or metastasis. Before diagnosis, 5 PSP patients (2.7%) presented with hemorrhage (>100 mL) during CT-guided percutaneous puncture of the lungs; all patients improved after hemostatic treatment, and no cases with chronic bleeding were observed.
Discussion
PSP and lung cancer manifest as pulmonary nodules. In some cases, clinicians will utilize CT-guided percutaneous lung puncture to collect pathological samples. Only a few cases of PSP have been diagnosed by cytology, as the diagnosis requires immunohistochemistry (5), and PSP can be easily misdiagnosed as lung cancer (6), which necessitates clarification via surgery. This study included 3 cases of preoperative pulmonary puncture cytology that indicated the presence of cancer cells (2 cases of adenocarcinoma and 1 case of non-small cell lung cancer); these cases were finally diagnosed as PSP after surgery.
PSP is more common in East Asian populations and is rare among Western populations (7). In this study, PSP was more common in women who were non-smokers (88.2%), which is consistent with previous reports (8), and PSP was found to occur often in the 40–70-year age group. In contrast, lung cancer was more common in men (69.0%) and was often found in the 60–70-year age group; moreover, most patients were smokers. PSP often occurred in the left lower lobe (32.6%), with a lesion size of 10–20 mm (48.7%), while lung cancer did not affect a particular area of the lungs and often presented with a tumor size over 30 mm. Common clinical manifestations included cough, sputum, and chest pain, while patients with PSP were more likely to be asymptomatic than patients with lung cancer. In addition, 16.0% of PSP patients presented with chest pain, which was also previously reported in the literature as a rare sign (9), and more lung cancer cases were accompanied with systemic manifestations (weight loss, hoarseness, and systemic pain) than PSP. PSP and lung cancer do not show specific differences in terms of overall clinical symptoms, and systemic symptoms can be used as a point of identification between the two diseases.
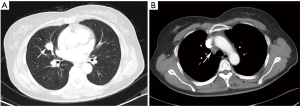
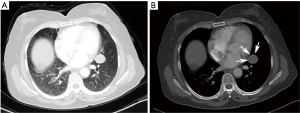
On the enhanced CT images before diagnosis, most of the lung cancer patients had lesions with rough boundaries or had short burrs on the lesion boundaries (89.3%), while 43.3% of the PSP patients also had lesions with the same unsmooth boundaries. In the study by Shin (10), 65.8% of the PSP patients had lesions with smooth boundaries, but fewer patients had lesions with unclear boundaries than in this study. PSP presented primarily as a single lesion without enlarged mediastinal lymph nodes, but 17.6% of patients also had lymphadenopathy (Figure 1). Pokharel (11) and Tanaka (12) also found that patients with PSP could have mediastinal lymph node invasion and thus have metastatic potential. In contrast, lung cancer showed no significant differences between single and multiple lesions and was usually accompanied by enlarged mediastinal lymph nodes (85.3%). In this study, one patient underwent EBUS-TBNA (Endobronchial ultrasound-guided transbronchial needle aspiration), and the 4R group lymph node biopsy suggested adenocarcinoma. This patient was diagnosed with lung adenocarcinoma and then underwent surgical treatment, but the surgical pathology examination revealed a diagnosis of PSP. Therefore, enlargement of the mediastinal lymph nodes cannot be considered as evidence of lung cancer and a misdiagnosis is still possible even after a cytology-based diagnosis. Yano (13) found that PSP was associated with contralateral lung metastasis, while this study also included a case of PSP with contralateral pulmonary nodules (right middle lobe + left lower lobe). Compared with the CT value of the muscle, the CT value of the PSP lesion was low (62.0%), and that of lung cancer was high (67.5%). PSP and lung cancer also showed a heterogeneous density within the lesion (54.6% and 75.1% for PSP and lung cancer, respectively) and no obvious calcification (75.9% and 81.2% for PSP and lung cancer, respectively). In addition, 64.5% of lung cancer patients showed lesions near the blood vessels or had vascular invasion, and 30.5% of PSP patients had lesions near the pleura or had pleural adhesions (Figure 2). Figure 3 was micrographs showing diagnostic characteristic of PSP. However, Devouassoux-Shisheboran (14) found that PSP lesions were essentially isolated with no adhesion (96%), which was different from the observation in this study. In addition to CT, FDG-PET (fluorodeoxyglucose positron emission tomography) can also be used to image PSP and lung cancer. However, limited numbers of reports have been published on FDG-PET imaging in cases of PSP (15,16). Some studies have shown that PSP also presents strong absorption in FDG-PET (17). However, these are only case reports, and whether FDG-PET can be used for PSP identification still requires the support of large numbers of cases.

Since PSP is rare in clinical practice, the number of cases accumulated in this study was small, and therefore, we need more time to accumulate additional patients for a more accurate data analysis.
Conclusions
PSP is a rare benign tumor that is difficult to diagnose clinically. It is often found in 40–70-year-old women who are non-smokers. It can be accompanied by cough and sputum, while a small number of patients experience chest pain. On CT imaging, PSP and lung cancer can both present as nodules or lumps, and PSP patients often show lesions with a size of 10–20 mm. The lesions are often solitary, located in the left lower lobe, and have unclear boundaries. Most patients do not present with enlargement of the mediastinal lymph nodes, and the CT value is low. It is difficult to differentiate between PSP and lung cancer, and this study can provide some basis for a differential diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine (ethics approval No. K17-137). All patients had signed an informed consent.
References
- Gaucher L, Patra P, Despins P, et al. A rare tumor: benign sclerosing pneumocytoma with an intrascissural development. Poumon Coeur 1983;39:321-6. [PubMed]
- Baysak A, Oz AT, Moğulkoç N, et al. A rare tumor of the lung: pulmonary sclerosing hemangioma (pneumocytoma). Respir Med 2013;107:448-50. [Crossref] [PubMed]
- Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956;9:53-75. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Dettrick A, Meikle A, Fong KM. Fine-needle aspiration diagnosis of sclerosing hemangioma (pneumocytoma): report of a case and review of the literature. Diagn Cytopathol 2014;42:242-6. [Crossref] [PubMed]
- Saha K, Sit NK, Jash D, et al. Diagnosis of sclerosing hemangioma of lung: don't rely on fine-needle aspiration cytology diagnosis alone. J Cancer Res Ther 2013;9:748-50. [Crossref] [PubMed]
- Zeng J, Zhou F, Wei XJ, et al. Sclerosing hemangioma: A diagnostic dilemma in fine needle aspiration cytology. Cytojournal 2016;13:9. [Crossref] [PubMed]
- Lim JH, Lee N, Choi DW, et al. Pulmonary sclerosing pneumocytoma mimicking lung cancer: Case report and review of the literature. Thorac Cancer 2016;7:508-11. [Crossref] [PubMed]
- Ruiz de la Cuesta D, Lafont Rufat M, Ruiz de la Cuesta Martín E. Pneumocytoma (formerly known as sclerosing hemangioma of the lung): a rare cause of chest pain. Arch Bronconeumol 2013;49:276-7. [PubMed]
- Shin SY, Kim MY, Oh SY, et al. Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltimore) 2015;94:e498. [Crossref] [PubMed]
- Pokharel S, Dhillon SS, Ylagan L, et al. Sclerosing Pneumocytoma with Lymph Node Metastasis. J Thorac Oncol 2016;11:1802-4. [Crossref] [PubMed]
- Tanaka I, Inoue M, Matsui Y, et al. A case of pneumocytoma (so-called sclerosing hemangioma) with lymph node metastasis. Jpn J Clin Oncol 1986;16:77-86. [PubMed]
- Yano M, Yamakawa Y, Kiriyama M, et al. Sclerosing hemangioma with metastases to multiple nodal stations. Ann Thorac Surg 2002;73:981-3. [Crossref] [PubMed]
- Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, et al. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000;24:906-16. [Crossref] [PubMed]
- Kamaleshwaran KK, Rajan F, Mehta S, et al. Multiple pulmonary sclerosing hemangiomas (pneumocytoma) mimicking lung metastasis detected in fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. Indian J Nucl Med 2014;29:168-70. [Crossref] [PubMed]
- Chen Q, Wu LJ, Hu H, et al. A case of pulmonary sclerosing hemangioma with low (18)FDG uptake in PET. Oncol Lett 2012;3:646-8. [PubMed]
- De Luca G, Martucci N, Setola S, et al. Sclerosing hemangioma of the lung mimicking pulmonary metastasis. Lung 2015;193:447-8. [Crossref] [PubMed]


