Transmanubrial osteomuscular sparing approach for resection of cervico-thoracic lesions
Introduction
Both benign and malignant lesions can affect the cervico-thoracic region, such as bronchogenic tumors, neurogenic tumors, soft-tissue neoplasms and metastasis from various primary sites (1-6). The incidence of these tumors is low, but when they appear, the crowded anatomy at the base of neck and thoracic inlet, the propensity of malignant tumor involving important structure and difficult access to this region make surgical resection quite a challenge (1,7,8).
In 1960s, Shaw and Paulson (6) introduced the posterior approach for Pancoast tumor, but this procedure was limited in dealing with lesions involving the subclavian vessels and it was reported with high morbidity and mortality. Afterwards, Dartevelle (1) described the anterior transclavicular approach which was able to provide a better exposure especially to the subclavian vessels and increase the resectability. But it was reported to be with cosmetic and functional disturbance of the shoulder. Since then there have been various modified anterior approaches, which basically derived from Dartevelle’s approach. We prefer the transmanubrial osteomuscular sparing approach (TMA) similar with Grunenwald’s (9) approach to preserve the sternoclavicular joint. In this article, we share our experience in applying the TMA and wanted to prove the feasibility and safety of the approach.
Methods
Population
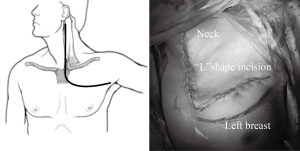
From April 2007 to January 2016, 58 cases with surgical resection of cervico-thoracic lesions via TMA (Figure 1) were found in our hospital. Signed consent forms were given by patients or their legal representative. After institutional review board approval, all the relative data were collected.
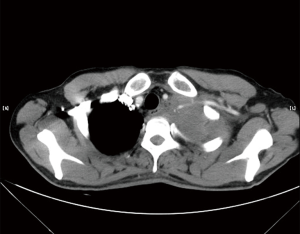
Careful preoperative assessments including cardiopulmonary function, resectability and metastasis were performed to ensure the patients were suitable for the operations. Chest computed tomography (CT) scan (Figure 2), abdominal ultrasound, brain CT scan and radionuclide bone scan were routines to exclude metastasis in bronchogenic carcinomas. PET-CT was also an option for the preoperative assessment in recent years. Bronchoscopy was given to the patients suspicious of bronchogenic carcinomas. Magnetic resonance imaging (MRI) was considered for lesions abut to the brachial plexus or subclavian vessels.
Surgical management and postoperative notes
We prefer a modified Grunenwald approach (9) for sparing the stemoclavicular joint. Once general anaesthesia with double-lumen intubation was done, the patient is placed in the supine position with neck hyperextended and turn away from the involved side. An “L” shape incision starts from the anterior edge of the sternocleidomastoid, with division of the upper sternum commonly into the 2nd intercostals space (Figure 1). Then the internal mammary artery is ligated and the first costal cartilage is resected, allowing the surgeon to lift the clavicle, the subclavian muscle, the transected part of the manubrium and the superior body of sternum. Subsequently, the subclavian vessels and its branches as well as brachial plexus can be exposed. The involved vein could just be resected while the affected portion of subclavian artery should be resected and reconstructed. The phrenic nerve should be carefully preserved if not involved. If T1 nerve root is taken up, then it should be divided proximal to the tumor at the level of T1 intervertebral foramen. Although tumor could extend superiorly into the brachial plexus, neurolysis could usually be achieved without division of any nerve roots above T1.
Perioperative complications and prevention
Adverse events occurring in hospital or within 30 days after surgery were defined as postoperative complications. Postoperatively, good analgesia, adequate pleural drainage, and aggressive pulmonary toilet are of great importance. The intravenous patient controlled analgesia (IV PCA) was used for a faster postoperative recovery. Individual cases may require special attention. After venous resection, arm elevation and massage would be helpful to reduce the edema. After artery reconstruction, routinely checking the pulse and extremity perfusion is required. For vascular resection or reconstruction patients, we usually start the low molecular weight heparin 4,100 IU every 12 h with hypodermic injection on the first postoperative day or when the chest drainage is stable, and change to oral aspirin when the patient is ambulatory. For tumors adjacent to or involving the brachial plexus, neurological function of the upper extremity should be checked after patients have regained their consciousness. For patients with hoarseness, precautions must be taken to avoid aspiration pneumonia and they may need temporary fasting or feeding via gastric tube for several days. After extensive chest wall resection, paradoxical breathing could be minimized by compression bandage.
Statistical analysis
Clinical characteristics and perioperative outcomes were analyzed using SPSS 19.0 software package (SPSS Inc., Chicago, IL, USA). Continuous variables were compared using two-sample t-tests. P<0.05 was considered to be statistically significant.
Results
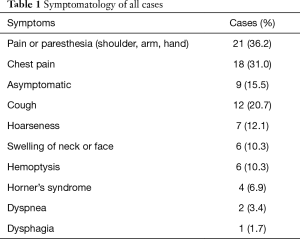
There were 22 neurogenic tumors, 21 bronchogenic tumors, and 15 other cases in the study, including 39 males and 19 females with mean age of 48.8 years (range 14–74 years). Nineteen patients were smokers, 8 were diabetic, 8 had hypertension, and 2 had coronary artery disease. In our series, the preoperative lung function showed: FEV1% was 85.30%±16.26% while DLCO% was 94.99%±17.93%. Eight of 21 bronchogenic tumor patients had neoadjuvant therapy and they were treated with platinum-based chemotherapy and received radiation at a dose of 30–45 Gy. Four patients had the surgery due to recurrence of primary disease (3 neurofibromatosis cases and 1 spindle cell malignant tumor case). The symptomatology was recorded in Table 1. The specific symptoms related to brachial plexus involvement were the most frequent.
Full table
There were 24 left and 34 right side procedures and there was no intraoperative and postoperative mortality. The average postoperative stay was 10.5 days (3–33 days). The mean operative time was 179.0 mins (57–328 mins). Nine (15.5%) patients, including 7 bronchogenic tumors, 2 neurofibromatosis cases, had massive bleeding (≥800 mL) during operation. Furthermore, the mean volume of blood loss for bronchogenic tumors was 900 mL, which was similar to non-bronchogenic tumors (474 mL, P=0.103). Moreover£¨patients with malignant tumors had more intraoperative blood loss than patients with benign diseases did (847 versus 194 mL, P=0.001).
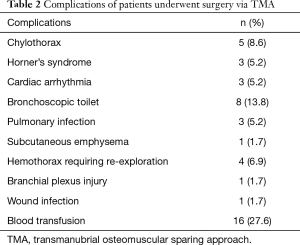
All the perioperative complications were presented in Table 2. Five (8.6%) cases were complicated with chylothorax and 2 of them had thoracic duct ligation procedure. All the chylothorax cases occurred in patients with left incision.
Full table
The origin of different tumors was listed in Table 3. Neurogenic tumors and bronchogenic carcinomas constitute the major component. There were 32 malignant cases and 26 benign cases. Twenty-eight of 32 (87.5%) malignant tumors had adjacent structure involvement. To achieve R0 resection, invaded adjacent structures were also resected (Table 4). Eight cases could not get R0 resection, including 5 bronchogenic tumors (malignant) and 3 cases of neurofibromatosis (benign). For malignant tumors, the average tumor size of incompletely excised cases (8.19±2.66 cm) was significantly larger than those completely resected (5.72±2.78 cm, P=0.023). For bronchogenic tumors, the mean lymph node stations dissected were 5 [1–12].
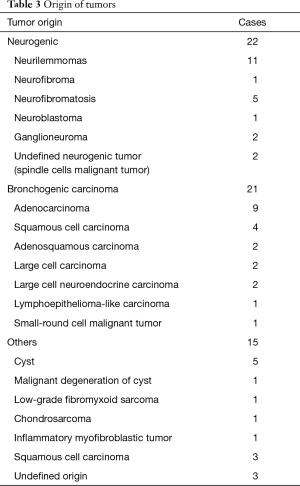
Full table
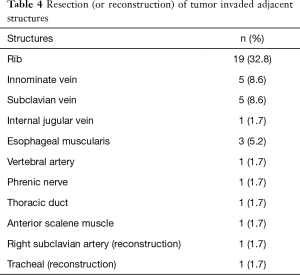
Full table
All 21 cases with branchial plexus compression symptoms were relieved after surgery. A separate patient suffered from postoperative motor dysfunction of the forearm due to branchial plexus injury. Three of 4 cases with preoperative Horner’s syndrome were relieved after surgery.
Discussion
Tumors involving the cervico-thoracic region used to remain no cure in early days. In 1960s, Shaw and Paulson (6) first introduced the posterior approach for resection of Pancoast tumor and that enlightened the surgical resection of cervico-thoracic tumor from different origins. But according to previous studies (5,10), the posterior approach could not provide adequate exposure to many important structures especially the subclavian vessels. This may be one of the reasons for the high incomplete resection rate, high mortality and morbidity rate in early reports (10-12). Later, Dartevelle first described the anterior transclavicular approach and it could provide excellent exposure to thoracic inlet. Even in cases of subclavian vessels infiltration, it can achieve an encouraging percentage of complete resection and acceptable percentage of morbidity and mortality (1,13). Over the last 20 years, a number of modified anterior approaches have risen to facilitate the resection of cervico-thoracic tumors (5,9,14,15).
In our practice, we also selected the anterior access for tumor in the anterior compartment or involving the subclavian vessels. TMA could ensure perfect preservation of the clavicle and the function of the sternocleidomastoid muscle, maintaining full shoulder joint function (16). In our study, all the cases were carried out in transmanubrial approach without additional incision. This was a large series of cases for assessing the single transmanubrial approach in resection of various cervico-thoracic lesions. Commonly, we selected the 2nd intercostal space to enter the chest cavity and conducted the resection. Some authors considered this access was limited for lobectomy, and they would like to add a posterolateral thoracotomy (5,17). In this study, all the upper lobectomy cases were finished through the 2nd intercostal space, except two upper sleeve lobectomy cases and one right upper-middle lobectomy case was done through 3rd intercostal space.
Due to the great exposure provided by the anterior approach, previous studies reported the remarkable improvement of resection rate and perioperative safety. Vanakesa (5) reported 22 cases of various cervico-thoracic tumors that had undergone surgical resection via the anterior-superior approach with no mortality and Spaggiari (18) also reported no mortality in their cases via TMA approach. Grunenwald and his colleagues (9) operated on 20 patients for cervico-thoracic lesions using classic anterior transcervical-thoracic approach (ATA) which led to shoulder dysfunction and aesthetic defects in most of the patients. In our series, there was also no perioperative mortality. In addition, unlike the transclavicular approach, all patients remained good shoulder performance and mobility postoperatively. We concluded that TMA is safe in dealing with tumors located in the cervico-thoracic region and can remain good functional and cosmetic effect of the shoulder. Compared to the posterior approach, anterior approach was reported with higher R0 resection rate. Here, sixteen of 21 bronchogenic tumor cases (76.2%) achieved R0 resection and our result was comparable to the previous reports (19-21).
There were some specific symptoms related to cervico-thoracic tumors. Compression or involving of brachial plexus could be indicated by pain, numbness, weakness or atrophy of the upper extremity, which were the commonest symptoms and 69.4% of the cases reported with those associated symptoms. Postoperatively, all the branchial plexus compression related symptoms were relieved. For advanced diseases that complete resection seemed to be impossible, neurolysis was still indicated for the relief of symptoms. Horner’s syndrome occurred in 4 of 58 cases and 3 patients were ameliorated postoperatively. There was one patient complicated with catastrophic motor dysfunction of forearm. So, we should be extremely careful during the dissection of nerve root. C8 should not be damaged to preserve the functional outcome of upper limb.
With regard to perioperative complications, 8 (13.8%) cases require bronchoscopic toilet while 7 of which with lung resection. Three (5.2%) cases were complicated with postoperative pneumonia. We considered that lung resection with extensive chest wall resection would compromise the postoperative respiration and effective expectoration. So compression bandage was routinely applied to such patients and would be helpful to the patients. Chylothorax occurred in 5 (8.6%) cases. Two cases failed conservative therapy and required ligation of the thoracic duct. All the chylothorax cases in our study occurred in patients with left incision due to the natural course of the thoracic duct. So in our latest left side cases, we would prophylactically ligate the thoracic duct when extensive resection is contemplated (Figure 3). We usually ligated the thoracic duct above the aortic arch, where it ascended along the left side of esophagus and vertebra.
Four of our patients, including 3 cases of neurofibromatosis and 1 case of neurogenic spindle cells malignant tumor, had operation due to recurrent disease and second operation was usually accompanied by severe adhesion and alteration of normal anatomy. Furthermore, the mean operative blood loss of re-operation due to recurrence was 1,400 mL. So great attention must be paid during dissection and there were no other solutions besides the surgeons’ personal experience, careful dissection and other meticulous preparation. Transfusion should be prepared preoperatively in case of massive bleeding during operation.
There are several limitations in this study. First, when compared to TMA, other surgical approaches remain scarce in our hospital, so we could not compare TMA with other surgical approaches. Second, although the study contained a relative large number of cases, it was still a single institutional study, which may cause potential bias. Third, with various pathological types, it was difficult to further investigate the relapse-free survival and overall survival.
In conclusion, TMA can be carried out safely in treating various cervico-thoracic lesions with good resection rate. It can avoid functional disability and cosmetic disturbance due to transclavicular approach. Left side procedure should be cautious of thoracic duct injury.
Acknowledgements
Funding: This work was funded by National Natural Science Foundation of China (Grant No. 81572245), Shanghai Rising Star Program (Grant No. 16QA1403500) and Shanghai Municipal Commission of Health and Family Planning (Grant No. 20144Y0169).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethics statement: The study was approved by institutional ethics committee of Shanghai Chest Hospital, Shanghai Jiao Tong University [No.KS(P)1713]. All the participants gave informed consent before taking part.
References
- Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025-34. [PubMed]
- Kraus DH, Huo J, Burt M. Surgical access to tumors of the cervicothoracic junction. Head Neck 1995;17:131-6. [Crossref] [PubMed]
- Nazari S. Transcervical approach (Dartevelle technique) for resection of lung tumors invading the thoracic inlet, sparing the clavicle. J Thorac Cardiovasc Surg 1996;112:558-60. [Crossref] [PubMed]
- Jaklitsch MT, Rego A. Endorsement for sparing the clavicle in the transcervical approach to the thoracic inlet. J Thorac Cardiovasc Surg 1997;113:959-61. [Crossref] [PubMed]
- Vanakesa T, Goldstraw P. Antero-superior approaches in the practice of thoracic surgery. Eur J Cardiothorac Surg 1999;15:774-80. [Crossref] [PubMed]
- Shaw RR, Paulson DL, Kee JL. Treatment of Superior Sulcus Tumor by Irradiation Followed by Resection. Ann Surg 1961;154:29-40. [Crossref] [PubMed]
- Millar J, Ball D, Worotniuk V, et al. Radiation treatment of superior sulcus lung carcinoma. Australas Radiol 1996;40:55-60. [Crossref] [PubMed]
- Pelton JJ, Ratner IA. Neuroblastoma of the thoracic inlet. J Pediatr Surg 1990;25:547-9. [Crossref] [PubMed]
- Grunenwald D, Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg 1997;63:563-6. [Crossref] [PubMed]
- Wright CD, Moncure AC, Shepard JA, et al. Superior sulcus lung tumors. Results of combined treatment (irradiation and radical resection). J Thorac Cardiovasc Surg 1987;94:69-74. [PubMed]
- Ginsberg RJ, Martini N, Zaman M, et al. Influence of surgical resection and brachytherapy in the management of superior sulcus tumor. Ann Thorac Surg 1994;57:1440-5. [Crossref] [PubMed]
- Okubo K, Wada H, Fukuse T, et al. Treatment of Pancoast tumors. Combined irradiation and radical resection. Thorac Cardiovasc Surg 1995;43:284-6. [Crossref] [PubMed]
- Lahon B, Mercier O, Fadel E, et al. Subclavian artery resection and reconstruction for thoracic inlet cancer: 25 years of experience. Ann Thorac Surg 2013;96:983-8; discussion 988-9. [Crossref] [PubMed]
- Dartevelle P, Macchiarini P. Surgical management of superior sulcus tumors. Oncologist 1999;4:398-407. [PubMed]
- Masaoka A, Ito Y, Yasumitsu T. Anterior approach for tumor of the superior sulcus. J Thorac Cardiovasc Surg 1979;78:413-5. [PubMed]
- Foroulis CN, Zarogoulidis P, Darwiche K, et al. Superior sulcus (Pancoast) tumors: current evidence on diagnosis and radical treatment. J Thorac Dis 2013;5 Suppl 4:S342-58. [PubMed]
- Marra A, Eberhardt W, Pöttgen C, et al. Induction chemotherapy, concurrent chemoradiation and surgery for Pancoast tumour. Eur Respir J 2007;29:117-26. [Crossref] [PubMed]
- Spaggiari L, Calabrese L, Gioacchino G, et al. Cervico-thoracic tumors resection through transmanubrial osteomuscular sparing approach. Eur J Cardiothorac Surg 1999;16:564-7. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol 2008;26:644-9. [Crossref] [PubMed]
- Sartori F, Rea F, Calabrò F, et al. Carcinoma of the superior pulmonary sulcus. Results of irradiation and radical resection. J Thorac Cardiovasc Surg 1992;104:679-83. [PubMed]