Four arm robotic-assisted pulmonary resection-right upper lobectomy: how to do it
Introduction
Since the first experience of robotic-assisted thoracoscopic surgery (RATS) for lung cancer reported by Melfi et al. in 2002 (1), robotic surgery has rapidly gained popularity among the international community of thoracic surgeons. Robotic lobectomy has been increasingly used for the treatment of early-stage non-small cell lung cancer (NSCLC), owing to its advanced features, including three-dimensional visualisation and wristed instrumentation, which facilitate complex movements even in the remote area of the chest cavity. However robotic surgery offers certain limitations such as the need for specialised operating room team and adequate surgeons training. Moreover, the multiple setup time and, the absence of haptic/tactile feedback and increased costs still represent an issue (2). In the last few years numerous authors have developed different technique/approach to perform a robotic lobectomy, reporting excellent results regarding intraoperative criteria and postoperative clinical and oncological outcome (3-5). Since the beginning of our experience we adopted the approach described by Veronesi in 2010 (6). This technique allows to proceed with an anterior to posterior approach to the hilum. We perform three ports (10–15 mm) and an anterior 3 to 4 cm utility incision.
In this study, we present, in sequential steps the four-arms robotic assisted technique to perform a robotic right upper lobectomy
Clinical vignette
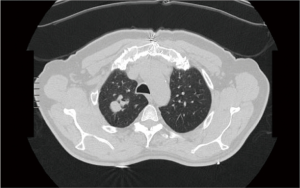
A 70 years old lady, former smoker, was admitted our division with a diagnosis of a 45 mm × 35 mm ground glass opacity located in the upper lobe of the right lung (Figure 1). The lady had a previous clinical history of a myocardial infarction and underwent coronary artery bypass graft surgery ten years earlier. The preoperative work-up included total body computer tomography, PET-CT-scan and a CT guided fine needle aspiration biopsy. Clinical stage was cT1N0M0 Adenocarcinoma. Patient was scheduled for a robotic right upper lobectomy. The operation was performed using the Da Vinci Si surgical system with a four arms approach (Figure 2).

Preference cards
- 3D high-definition camera (30° lens).
- 1 Fenestrated Bipolar Forceps: this forceps allows dissection and coagulation and is very useful to speed-up the procedure.
- 2 Cadiere Forceps: the Cadiere Forceps help to gently retract the lung parenchyma and should be used to perform a safe blunt dissection around vascular adventitia.
- Permanent Cautery Hook (EndoWrist Monopolar Cautery).
- Endo GIA™ 30 mm Curved Tip Articulating Vascular/Medium Reload with Tri-Staple™ Technology (Covidien): the curved tip facilitates to slide the stapler around the vessels safely.
- Endowrist 8 mm clip appliers (Medium-large clips): it should be used to facilitate isolation and dissection of small pulmonary artery branches (up to 8 mm).
- Energy device: harmonic ACE ultrasonic.
- Soft tissue retractor: we usually use AlexisTM (Applied Medical).
- Silicone vessel loops.
Surgical technique
Patient positioning
The procedure is performed under general anaesthesia. Patient is placed in left lateral decubitus with the chest orientated parallel to the floor. To achieve maximum separation of the intercostal spaces the hips should be fixed at the level of the table break and flexed. The Si da Vinci robot is finally positioned at the head of the patient.
Port placement
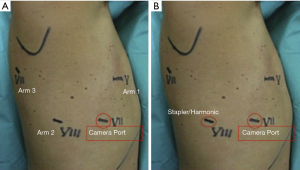
We always start the procedure realising a 3-cm utility incision at the fifth intercostal space anteriorly to the board of the latissimus dorsi. We explore the pleural cavity with a 10 mm 30° VATS camera. All the other ports are performed under view guidance. The utility incision is generally protected by a soft tissue retractor. The 12 mm camera port is placed at the level of the mid-axillary line in the seventh intercostal space. Two operative ports are performed respectively in the eighth intercostal space posteriorly and in the auscultatory triangle. We complete the set-up docking the Robot. Usually port placement and robot set up takes from 5 to 8 minutes. The 30-degree stereoscopic robotic camera, is then introduced and under direct view we begin putting the three operative robotics arms. To retract the lobe and achieve a better exposure of the hilum we use a Cadiere forceps inserted through the fourth posterior trocar (arm 3). The permanent cautery hook is placed at the level of the utility incision (surgeon right hand: arm 1) and the fenestrated bipolar forceps in the other operative port (surgeon right hand; arm 2) (Figure 3).
Robotic right upper lobectomy
Step 1: inspection and pleural adhesions lysis
After a careful inspection of the pleural surface, to confirm the absence of pleural effusion or metastasis, we start the procedure. Pleural adhesions may offer a technical challenge even though not represent per se a contraindication to Robotic surgery. The key is to identify the correct pleural cavity plane and create space to perform adhesion lysis. We usually deliver a combination of blunt and sharp dissection. The use of the permanent cautery hook may facilitate dissection of dense adhesions, speeding up the procedure. A sponge mounted on the cadiere forceps helps to retract the lung and identify the correct pleural plane avoiding the direct grasp of the parenchyma that could be easily torn.
Step 2: right upper lobe vein
We proceed with an anterior to posterior approach to the hilum. With the arm 3 (cadiere forceps) we retract the upper lobe posteriorly and gently pull it towards the apex. With the cautery hook, we open the pleura anteriorly to the upper lobe vein parallel to the course of the phrenic nerve all the way down to expose the lower lobe vein and confirm the absence of anomalies of the venous anatomy. Once the bifurcation between the middle and upper lobe vein is identified the upper vein is isolated, encircled with a vessel loop and finally divided with stapler (30 mm vascular reload) introduced from the posterior (Figure 3A).
Step 3: PA branches identification and isolation
Now, the upper lobe should be pulled posteriorly and inferiorly with the Cadiere forceps (arm 3). This manoeuvre allows a clear visualisation of the main pulmonary artery. The mediastinal pleura is sectioned around the hilum, lateral to the superior vena cava, following the inferior margin of the azygos vein, going posteriorly over the bronchus. Blunt dissection of the adventitial plane is completed and the first PA branch (one or two branches, usually) is exposed, isolated, and divided with a vascular 30 mm reload introduced again from the posterior port (Figure 3B). Proceeding with a blunt dissection over the top of the PA, the posterior ascending PA branch (for the segment S3 of the upper lobe) should be easily identified. The isolation of this branch, from an anterior approach, can be challenging. The posterior branch has a course close and parallels to the right upper bronchus, and frequently there is no a good angulation to slide a stapler around it. Moreover, is usually a small branch and can be easily injured. We usually prefer to divide it placing one or two hemlock clips proximally and separate it with the harmonic device.
Step 4: right upper lobe bronchus
The lobe should be pulled anteriorly and toward the apex to expose the right upper bronchus. Posterior mediastinal pleura is then dissected. At this time all hilar lymph nodes should be removed to facilitate isolation of the bronchus. The stapler (a 60 mm reload) is usually introduced by the bed-assistant trough the utility incision, however can be inserted through the posterior port (arm 2) if it offer a better angle. We complete the procedure dividing the fissure introducing the stapler (60 mm reload) from the utility incision.
We then pull the lobe out of the chest with a specimen retrieval bag through the utility incision. Overall the procedure timing (docking, lobectomy and lymph nodes dissection) was 112 minutes.
Step 5: lymph node dissection
- Station 7 (subcarinal): the lower lobe should be pulled towards the apex so to expose the inferior pulmonary ligament. The bed-assistant should introduce a sponge stick from the utility incision to push the diaphragm down improving visualisation of the target area. With the hook diathermy (arm 1) we start dissecting the ligament all the way up, removing all the lymph node (stations 8 and 9). The remaining lobe is then retracted anteriorly and superiorly. Posterior pleura should be open from the superior edge of the lower lobe vein, along with the course of the vagus nerve. This manoeuvre allows a clear identification of the target area and a safe visualisation of the oesophagus.
- We usually approach the paratracheal area dissecting the mediastinal pleura under the azygos vein and along the lateral profile of the superior vena cava. The mediastinal pleural is then retracted posteriorly with Cadiere forceps (arm 3). Stations 2R and 4R that are removed en bloc.
Discussion
- The fourth arm should be used for lung retraction to improve exposure of the operative field
- We always have a rolled-up sponge available on the operative field when working on vascular structures and, as shown in this video case, to facilitate dissection of pleural adhesions.
- Some authors suggested the isolation and division of the right upper bronchus before proceeding with the separation of the posterior ascending branch. According to our experience, we recommend first to divide the posterior branch. This should facilitate identification of the inferior edge of the right upper bronchus and its safe isolation.
- The procedure described is a “fissure last” technique. If this approach does not offer a “safe” angulation to slide a stapler or to clip the posterior branch of segment S3, a division of the fissure should be considered to isolate the S3 branch from a posterior view.
- The Endowrist clip appliers should be considered for small PA branches (7 or less mm). We usually place one or two clips on the proximal end, and we divide the distal one with the Harmonic ultrasonic energy.
Conclusions
As shown above, via the operative techniques, pictures, and video the four-arm approach offers an outstanding visibility of the hilar structures during robotic surgery. This is a feasible and reproducible technique and appears to be well suited to perform a lung lobectomy safely.
The utility incision (3 to 4 cm) provide enough to space to introduce one robotic arm together with the instruments controlled by the bed assistant, such as sucker, sponge stick and stapler. Moreover, in the case of uncontrolled bleeding can be converted rapidly to lateral thoracotomy.
To safely find the right angle to slide the stapler around pulmonary artery and/or vein we usually encircle pulmonary vessels with a vessel loop and we prefer to use curved tip articulated vascular reload (Tri-Staple™ Technology Covidien).
The future of robotic surgery is ravishing. Multiple new instruments will soon be available to make safer and more efficient procedures. There are even new robotic platforms being developed. Thoracic surgeons around the world must accept to deal with robotic technology. The Robot is here and is going to stay because as reported by Leonardo da Vinci: “Human subtlety will never devise an invention more beautiful, simpler or more direct than does nature because in her inventions nothing is lacking, and nothing is superfluous”.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Hernandez JM, Humphries LA, Keeling WB, et al. Robotic lobectomy: fattening the learning curve. J Robot Surg 2012;6:41-5. [Crossref] [PubMed]
- Ninan M, Dylewski MR. Total port-access robot assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Pardolesi A, Bertolaccini A, Brandolini J, et al. Edited right upper lobectomy (robot daVinci Si). Asvide 2017;4:422. Available online: http://www.asvide.com/articles/1736


