Surgery for a large tracheoesophageal fistula using extracorporeal membrane oxygenation
Introduction
Fistula formation between the esophagus and the tracheobronchial tree after definitive chemo-radiotherapy for esophageal cancer is a life-threatening complication. Conservative, endoscopic and surgical repair of the fistula has been advocated. The choice for a treatment is directed by the location and size of the fistula as well as patient’s condition. If surgery is indicated, the management of the airway and ventilation can be a major challenge. Here, we present a patient who underwent an extensive surgical treatment of a massive tracheoesophageal fistula (TEF) by using veno-venous extracorporeal membrane oxygenation (VV-ECMO).
Case presentation
A 72-year-old man underwent definitive chemoradiation (50 Gy with carboplatin/paclitaxel) for a cT4bN1M0 squamous cell carcinoma of the mid esophagus with complete remission. He developed a symptomatic TEF, which was treated with esophageal self-expandable metal stents. With the esophageal stent he was asymptomatic and therefore refused surgery given his excellent quality of life. However, over the years, due to multiple stent placements the TEF slowly increased in size and teared out the proximal esophagus, which limited stent placement as a treatment option. At last a fully covered self-expandable metal stent of 17 cm in length and 18 mm in diameter was required to cover the entire defect. The upper end of the stent was situated just below the upper esophageal sphincter (UES) and the lower end just covered the defect that ran to the carina (Figure 1). This situation introduced a significant potential risk of life threatening acute airway obstruction. Furthermore, the patient suffered from recurrent pneumonias and enteral nutrition was given via a nasojejunal feeding tube. Endoscopic placement of yet another stent was considered technically impossible, leaving surgery as the only curative option. The patient had no history of any cardiovascular disease. Because of his very good performance state we found him fit for surgery. Mechanical ventilation with selective lung ventilation techniques was considered not feasible given the extension of the TEF to the carina. Positive pressure ventilation was contraindicated during the entire procedure; preoperative as this could lead to gastric inflation, during surgery due to disturbing the surgery site and postoperative as this could damage the anastomosis. Therefore, to secure ventilation and oxygenation VV-ECMO was considered to be the best option.
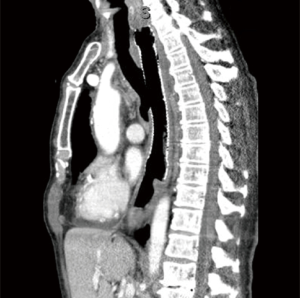
VV-ECMO was initiated with esketamine propofol sedation while spontaneous ventilation was maintained. To ensure the possibility of high ECMO blood flow, two cannulas were used; the right femoral vein was cannulated with a 25 Fr cannula (length 55 cm) for inflow. For the outflow, the right internal jugular vein was cannulated with a 21 Fr cannula (length 14 cm). The femoral cannula was positioned with the tip 1 cm in the right atrium under transthoracic echocardiographic guidance. As the returning cannula was so short, we assumed the distance to the drainage cannula to be more than 10 cm and would result in a low recirculation. These cannulae were connected to the ECMO system, with a maximal blood flow capacity of 7 L/min (iLa active XLung kit, NovaLung, Heilbron, Germany). A bolus of 5,000 units heparin was administered while ECMO support was initiated without continuous heparin administration. As soon as adequate ventilation and oxygenation with the ECMO system was confirmed, anesthesia was initiated, without tracheal intubation. Firstly, a fibro-bronchoscopy confirmed adequate position of the covered stent in the esophagus without migration to the trachea. Next, the stent was removed by gastroscopy (Figure 2). After this, a large tracheoesophageal defect was revealed where no posterior wall of the trachea remained, starting just below the larynx and the UES, and ending at the level of the carina (Figures 2,3). With a left anterior neck incision, the cervical esophagus was dissected and uncirculated. An endostapler was used to close and divide the esophagus just below the UES, but leaving the TEF intact. Via a laparotomy the distal esophagus was mobilized and transected with an endostapler at the level of the pulmonary vein, thereby leaving a short blind ending distal esophagus. The excluded native esophagus formed the new posterior wall of the trachea. A gastric tube was brought up to the neck retrosternal and a hand sown anastomosis was performed between the conduit and the esophageal stump (Figure 4). Finally, a feeding jejunostomy was created and a surgical tracheostomy was placed. A fibro-bronchoscopy was performed to remove blood and secretion and showed that all lung areas were open. After discontinuation of total intravenous anesthesia and reversal of muscle relaxation, the patient was weaned from ECMO and after confirming adequate ventilation and oxygenation, decannulated and transferred to the ICU. Total time of surgery was 415 minutes and total blood loss was 1,500 mL. Total ECMO time was 565 minutes, including 530 minutes of apnoea. During the entire ECMO run we achieved a mean ECMO blood flow of 5.1 (range, 4.5–5.5) L/min without significant cavitation of the return cannula. Serial arterial blood gas analyses showed acceptable oxygenation during the entire ECMO run (PO2 range, 7.0–11.0 kPa) and showed adequate carbon dioxide elimination achieved with a sweep gas flow of 3 to 4 L/min.

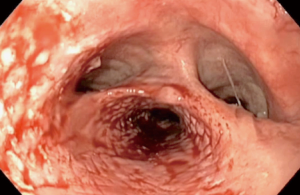
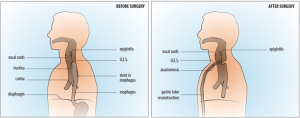
The patient was observed in the ICU for two days. He was discharged from the hospital at day 18, without any cardiopulmonary adverse events. One year after surgery, he is in excellent general health without evidence of recurrent or metastatic disease.
Discussion
We describe for the first time successfully application of VV-ECMO during a 7-h apnoeic surgery. The aim of the operation was to reconstruct the trachea and restore the passage of food. Given the destruction of a large part of the membranous part of the trachea and the extent of the TEF, a reconstruction of the membranous part of the trachea would have been a major challenge. Secondly, the radiation made us reluctant to perform a local reconstruction. The longstanding inflammatory reaction due to the radiation and stents had created a fibrotic esophagus that served as an autologous membranous part of the trachea. An operation using the esophagus to repair a large TEF was originally described by Bartlett et al. (2).
Positive pressure ventilation was impossible, even selective ventilation. By choosing VV-ECMO, cardiopulmonary bypass with central cannulation via thoracotomy was prevented reducing pulmonary morbidity and making high dose systemic anticoagulation unnecessary. Perioperative use of veno-arterial (VA)-ECMO has been described first in paediatric and later in adult patients and is predominantly used for surgery of complex mediastinal tumours, lung and airway. It has also been used to facilitate surgery for TEF (3). As VA-ECMO can replace heart and lung function it may be useful to assist oxygenation during surgery in patients who are also at risk of right heart failure in case of sudden significant increased right ventricular afterload. A disadvantage of VA-ECMO in surgery when apnoea is required, however, is the phenomenon of differential hypoxia, since hypoxic blood returning from the pulmonary vein is ejected by the left heart (4,5). In our patient, we did not expect a significant increase in right ventricular afterload as pulmonary vasculature was not to be compromised and we therefore chose the veno-venous mode because this mode does not have the drawback of differential hypoxia.
The present case demonstrates VV-ECMO can successfully facilitate apnoeic surgery for a considerable time. The use of VV-ECMO has been described for procedures with a relatively short period of apnoeic ventilation. As apnoeic ventilation becomes longer, the risk of desaturation increases due to various factors. For instance, the inability to maintain high ECMO blood flow due to surgical bleeding or increased cardiac output caused by surgical trauma with a systemic inflammatory response. Therefore, in some comparable cases (3), authors deliberately chose VA-ECMO. They considered that VV-ECMO could not achieve stabile arterial oxygen saturations for a longer period of time. This assumption was based on the risk of considerable recirculation and the need to maintain a relative high VV-ECMO blood flow of more than 60% of the patients’ cardiac output to achieve an arterial oxygen saturation of 90% or more (6). Collins et al. and Pinelli et al. did use the veno-venous mode of ECMO to support an extensive TEF repair. However, in the first case the oxygenation was augmented by supplemental high flow apnoeic oxygen delivery using a breathing circuit (7) and in the second case by using VV-ECMO by a jugular Avalon catheter as adjunct to minimal mechanical ventilation (8). Using the native lung for oxygenation was not possible in our patient because this would disturb the surgical field.
In conclusion, this case shows VV-ECMO support can be a successful strategy to perform a 7-h lasting surgery without the use of positive pressure ventilation at any time. Based on a growing number of case reports, it is conceivable that ECMO will be used more often to facilitate complex surgery, in which positive pressure ventilation is contraindicated, even when long periods of apnoea are expected.
Acknowledgements
We thank Dr. CA Meeuwis (Department of Otolaryngology Head and Neck Surgery).
Footnote
Conflicts of Interest: D Dos Reis Miranda has received honaria from NovaLung (Heilbronn, Germany) for giving lectures. The other authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- van Drumpt AS, Kroon HM, Grüne F, et al. Preoperative endoscopy demonstrating the position of the esophageal stent and demonstrating the large trachea-esophageal fistula reaching the carina after stent removal. Asvide 2017;4:423. Available online: http://www.asvide.com/articles/1737 .
- Bartlett RH. A procedure for management of acquired tracheoesophageal fistula in ventilator patients. J Thorac Cardiovasc Surg 1976;71:89-95. [PubMed]
- Wang L, Xu XP, Zhan H, et al. Application of ECMO to the treatment of benign double tracheoesophageal fistula: report of a case. Ann Thorac Cardiovasc Surg 2014;20 Suppl:423-6. [Crossref] [PubMed]
- Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol 2016;105:283-96. [Crossref] [PubMed]
- Rupprecht L, Lunz D, Philipp A, et al. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel 2015;7:320-6. [PubMed]
- Schmidt M, Tachon G, Devilliers C, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 2013;39:838-46. [Crossref] [PubMed]
- Collins NF, Ellard L, Licari E, et al. Veno-venous extracorporeal membrane oxygenation and apnoeic oxygenation for tracheo-oesophageal fistula repair in a previously pneumonectomised patient. Anaesth Intensive Care 2014;42:789-92. [PubMed]
- Pinelli F, Romagnoli S, Bevilacqua S, et al. Extracorporeal membrane oxygenation-assisted esophagectomy. J Cardiothorac Vasc Anesth 2015;29:436-8. [Crossref] [PubMed]


