Therapeutic procedure in small cell lung cancer
Introduction
Lung cancer is one of the first cause of cancer death in the USA (1). First time described and identified in 1959, as a lung cancer with clinical and overall survival prognosis significantly different from non-small cell lung cancer (NSCLC), who was more common till this year. The proportion of small cell lung cancer (SCLC; among all lung cancer histological types) decreased from 17.26% in 1986 to 12.95% in 2002 (2). The proportion of women with SCLC increased from 28% in 1973 to 50% in 2002 (3). Further results after then are poor First line chemotherapy based in cisplatin and remain the only treatment for extensive SCLC who increase overall survival with the same toxicity (4). SCLC has high rate of initial response to chemotherapy (60-70%) in patients with extensive disease but mean survival is not better than 10 months because 70% of patients with SCLC are diagnosed with advanced disease with aggressive clinical course. This review focuses on the therapeutic procedure and management of SCLC.
Search method
Our search strategy for the selection of articles is described below. We searched the Medline databases for articles published between January of 1996 and October of 2013. In addition, we searched The Physician Data Query database for clinical trials and the proceedings of the American Society of Clinical Oncology, the American Society for Therapeutic Radiology and Oncology [1992-2013], the European Society of Therapeutic Radiology and Oncology [2000-2013], and the European Society for Medical Oncology [1998-2013] for relevant abstracts. Relevant articles and abstracts were selected and reviewed and the corresponding lists of references were scanned for additional studies.
Staging system
We have two systems to stage SCLC: (I) The tumour-node-metastases (TNM) classification (5), that used for NSCLC; and (II) The VA Lung Study Group (VALSG) limited disease- extensive stage (LD-ED) system. The Veterans’ Administration Lung Study Group (VALSG) two-stage classification scheme has been routinely used for the clinical staging of SCLC since the late 1950s (6). The VALSG system defines limited-stage (LS) as: (I) disease confined to one hemithorax, although local extension may be present; (II) no extrathoracic metastases except for ipsilateral supraclavicular lymph nodes if they can be included in the same radiation port as the primary tumor; and (III) primary tumor and regional nodes that can be adequately encompassed in a radiation port. Extensive-stage (ES) disease is defined as disease that cannot be classified as limited, including malignant pleural or pericardial effusions, contralateral hilar or supraclavicular lymph nodes, and hematogenous metastases. In 1989 (7), the International Association for the Study of Lung Cancer (IASLC) proposed a modification of the VALSG system in which LS-SCLC was expanded to include contralateral mediastinal or supraclavicular lymph node metastases and ipsilateral pleural effusions independent of cytology (8). ES-SCLC remained any disease at sites beyond the definition of limited disease. Although the IASLC system has a higher discriminatory power (9), the VALSG system continues to be widely utilized, probably because of its simplicity. Recently, the IASLC has proposed that the newly revised TNM staging classification for lung cancer [American Joint Committee on Cancer (AJCC) 7th edition] (10) should replace the VALSG system for the staging of SCLC. This recommendation is based on a prognostic analysis of 8,088 patients with SCLC in the IASLC database with adequate data to determine clinical (c) or pathologic (p) TNM stage (11,12). Many trials for LD exclude patients with isolated pleural effusions (13-15), but overall survival of patients with pleural effusions is approximately same to other patients with LD-SCLC (16,17). Supraclavicular lymph node metastatic disease, may predict for inferior survival (18,19).
Therapeutic management
The gold standard first line chemotherapy as treatment of limited-stage SCLC is cisplatin plus etoposide in parallel with thoracic radiation therapy, but treatment of extensive-stage disease is only chemotherapy with cisplatin plus etoposide. Surgical resection reserved for patients with small, node-negative disease staged as a very limited disease. Prophylactic cranial irradiation (PCI) reduces possibilities of brain metastases and prolongs overall survival in patients who have responded to chemotherapy.
Chemotherapy
1st line chemotherapy
SCLC is more chemosensitive (20) than all other types of lung cancer. First trials in the 1970s tried effectiveness of cyclophosphamide, doxorubicin/epirubicin and vincristine [CA(E)V] in SCLC (21-23). But after introduction of etoposide, comparing etoposide-cisplatin (EP) with CA(E)V found EP inferiority with better results about disease free and overall survival in patients with limited stage disease. The response rates were higher with EP in patients with ED, but without a survival benefit (24,25). After then, EP is better tolerated as the regimen of choice for initial treatment of SCLC (26).
Carboplatin in many trials is used instead of cisplatin in combination with etoposide (27) without differences in response rates, but significantly less toxicity (28). In clinical practice, with carboplatin reduce the risk of emesis, neuropathy, and nephropathy. The use of carboplatin has a greater risk of myelosuppression than the use of cisplatin. Additioning paclitaxel with cisplatin or carboplatin plus etoposide promised similar results in phase II trials but did not improve survival, and has association with unacceptable toxicity in a phase III study (29). Using maintenance or consolidation chemotherapy 4 to 6 cycles of standard treatment created a minor prolongation of duration of response but without improving overall survival and with greater risk of toxicity (30,31). The combination of irinotecan and a platinum agent has provided the greatest challenge to EP. A phase III trial performed in Japan found that patients with extensive-stage SCLC who were treated with irinotecan plus cisplatin had a median survival of 12.8 months, greater than 9.4 months for patients who treated only with EP (32). Till now, the community continues to recommend etoposide plus platinum as the standard regimen for patients with SCLC.
2nd line chemotherapy
At present, topotecan is the only drug approved by the US Food and Drug Administration for relapsed SCLC, and is considered the standard second-line chemotherapy in many countries. More recently, amrubicin has also shown more favorable antitumor activity, and is the most promising at present.
Topotecan is proposed as monotherapy for patients with relapsed SCLC with SD or PD after first line chemo-radiotherapy. For Topotecan the first positive opinion came from the “Committee for Human Medicinal Products (CHMP) on 24 January 2008” as monotherapy for the treatment of adult patients with relapsed SCLC for whom re-treatment with the first line regimen is not considered appropriate (30). Data from phase II studies suggested that amrubicin, an anthracycline, promised activity in patients with relapsed or refractory SCLC with the most common problem of grade 3/4 toxicity, primarily neutropenia (33,34). A randomized phase II trial suggested that amrubicin could be more effective than topotecan as second-line therapy in patients with relapsed SCLC, with response rates of 44% and 15%, respectively (P=0.02) (35,36).
Thoracic radiation
Most of patients with LD-SCLC treated only with chemotherapy. However, thoracic radiation therapy (TRT) can provide local control. In the other side cannot improve results on overall survival disease control (37). Many randomized trials tried to combine them to achieve better overall disease control. Although chemotherapy achieves high response rates, its use alone is associated with fairly high intrathoracic recurrence rates. Thoracic irradiation at doses not inferior to 40 Gy can induce local response, but by itself is unable to achieve good disease control. Combination therapy, consisting of thoracic irradiation and chemotherapy, produced better survival than chemotherapy alone in some trials (38,39) although other trials using cyclophosphamide-based therapy failed to show a survival benefit when irradiation was added (40).
The National Cancer Institute of Canada found that patients who received more than 37.5 Gy (Gray) had a better local control than those who received less than 25 Gy (40), but without better results in overall survival. An analysis of patients in three different dose chemoradiation trials, who treated with 45, 55 and 65 Gy found similarity in local control of disease and overall survival with the three doses analyzed. Suggested a dose at least 45 Gy for adequation local control (41).
The most commonly utilized fractionation schedules suggest single daily treatments of 1.8 to 2.0 Gy, five times per week, over 5 to 6 wks. Hyperfractionated radiotherapy found that improves local control and survival by using higher doses of radiation given in a shorter time. A randomized phase III trial (41), showed that patients who received the accelerated twice-daily schedule had better median survival (23 vs. 19 months), and 5-year overall survival (26% vs. 16%). A meta-analysis (41) suggested that there was no difference between early or late TRT on overall survival and there was a significantly improved 5-year overall survival with early TRT.
PCI
Brain metastasis is very common in patients with SCLC. Approximately 25 percent of patients have brain metastases at the first diagnostic procedure (42). A lot of trials evaluate the role of PCI in SCLC (43,44) with variation in their findings. PCI is recommended for patients with extensive-stage disease with a complete or partial response (45,46). The recommended regimens for PCI include not less than 24 Gy per day (46). Higher doses (e.g., 36 Gy) increased mortality and toxicity when compared with standard doses (25 Gy) (47). PCI should not be given concurrently with systemic chemotherapy, and high total radiotherapy dose (>30 Gy) should be avoided because of the increased risk of neurotoxicity. Fatigue, headache, and nausea/vomiting are the most common acute toxic effects after PCI (48).
Surgery
SCLC is considered as a systemic disease, and the role of surgery in the management of these patients (49-51) not exist in clinical practice. However, recent studies showed better results for surgical resection but only in early stage disease (very limited disease) (52). In contrast, there is currently no role for resection in the multimodality treatment of locally advanced SCLC.
Palliative treatment
Radiotherapy can provide excellent palliation for patients with localized symptomatic sites of disease (e.g., painful bony lesions, spinal cord compression, and obstructive atelectasis) or with brain metastases (53-55). Orthopedic stabilization may be useful in patients at high risk for fracture because of osseous structural impairment.
Future
Targeted biological therapies for SCLC are now being investigated, and although a great deal of research remains to be done, these agents may provide the hope for future treatment of SCLC (Table 1).
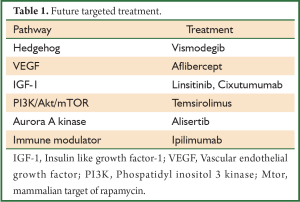
Full Table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- International Agency for Research on Cancer [homepage on the Internet]. Lyon: International Agency for Research on Cancer [cited 2006 Jul 20]. Globocan 2002.
- Cohen MH, Matthews MJ. Small cell bronchogenic carcinoma: a distinct clinicopathologic entity. Semin Oncol 1978;5:234-43. [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [PubMed]
- Le Péchoux C, Dhermain F, Bretel JJ, et al. Modalities of radiotherapy in small cell lung cancer: thoracic radiotherapy and prophylactic cerebral irradiation. Rev Pneumol Clin 2004;60:3S91-103.
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [PubMed]
- Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3 1973;4:31-42. [PubMed]
- Stahel RA, Ginsberg R, Havemann K, et al. Staging and prognostic factors in small cell lung cancer; a consensus report. Lung Cancer 1989;5:119-26.
- Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease? Lung Cancer 2002;37:271-6. [PubMed]
- Argiris A, Murren JR. Staging and clinical prognostic factors for small-cell lung cancer. Cancer J 2001;7:437-47. [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Handbook. 7th ed. New York: Springer, 2010:299-323.
- Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067-77. [PubMed]
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [PubMed]
- Perry MC, Eaton WL, Propert KJ, et al. Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med 1987;316:912-8. [PubMed]
- Work E, Nielsen OS, Bentzen SM, et al. Randomized study of initial versus late chest irradiation combined with chemotherapy in limited-stage small-cell lung cancer. Aarhus Lung Cancer Group. J Clin Oncol 1997;15:3030-7. [PubMed]
- Bunn PA Jr, Lichter AS, Makuch RW, et al. Chemotherapy alone or chemotherapy with chest radiation therapy in limited stage small cell lung cancer. A prospective, randomized trial. Ann Intern Med 1987;106:655-62. [PubMed]
- Dearing MP, Steinberg SM, Phelps R, et al. Outcome of patients with small-cell lung cancer: effect of changes in staging procedures and imaging technology on prognostic factors over 14 years. J Clin Oncol 1990;8:1042-9. [PubMed]
- Livingston RB, McCracken JD, Trauth CJ, et al. Isolated pleural effusion in small cell lung carcinoma: favorable prognosis. A review of the Southwest Oncology Group experience. Chest 1982;81:208-11. [PubMed]
- Spiegelman D, Maurer LH, Ware JH, et al. Prognostic factors in small-cell carcinoma of the lung: an analysis of 1,521 patients. J Clin Oncol 1989;7:344-54. [PubMed]
- Urban T, Chastang C, Vaylet F, et al. Prognostic significance of supraclavicular lymph nodes in small cell lung cancer: a study from four consecutive clinical trials, including 1,370 patients. “Petites Cellules” Group. Chest 1998;114:1538-41. [PubMed]
- Morstyn G, Ihde DC, Lichter AS, et al. Small cell lung cancer 1973-1983: early progress and recent obstacles. Int J Radiat Oncol Biol Phys 1984;10:515-39. [PubMed]
- Livingston RB, Moore TN, Heilbrun L, et al. Small-cell carcinoma of the lung: combined chemotherapy and radiation: a Southwest Oncology Group study. Ann Intern Med 1978;88:194-9. [PubMed]
- Feld R, Evans WK, DeBoer G, et al. Combined modality induction therapy without maintenance chemotherapy for small cell carcinoma of the lung. J Clin Oncol 1984;2:294-304. [PubMed]
- Feld R, Pringle JF, Evans WK, et al. Combined modality treatment of small cell carcinoma of the lung. Arch Intern Med 1981;141:469-73. [PubMed]
- Feld R, Evans WK, Coy P, et al. Canadian multicenter randomized trial comparing sequential and alternating administration of two non-cross-resistant chemotherapy combinations in patients with limited small-cell carcinoma of the lung. J Clin Oncol 1987;5:1401-9. [PubMed]
- Fukuoka M, Furuse K, Saijo N, et al. Randomized trial of cyclophosphamide, doxorubicin, and vincristine versus cisplatin and etoposide versus alternation of these regimens in small-cell lung cancer. J Natl Cancer Inst 1991;83:855-61. [PubMed]
- Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282-91. [PubMed]
- Skarlos DV, Samantas E, Kosmidis P, et al. Randomized comparison of etoposide-cisplatin vs. etoposide-carboplatin and irradiation in small-cell lung cancer. A Hellenic Co-operative Oncology Group study. Ann Oncol 1994;5:601-7. [PubMed]
- Bishop JF, Raghavan D, Stuart-Harris R, et al. Carboplatin (CBDCA, JM-8) and VP-16-213 in previously untreated patients with small-cell lung cancer. J Clin Oncol 1987;5:1574-8. [PubMed]
- EMEA Report-Scientific Discussion; Procedure No. EMEA/H/C/123/II/34; EMEA, London, 6 January 2006. Available online: , cited 2-6-2009.
- Niell HB, Herndon JE 2nd, Miller AA, et al. Randomized phase III intergroup trial of etoposide and cisplatin with or without paclitaxel and granulocyte colony-stimulating factor in patients with extensive-stage small-cell lung cancer: Cancer and Leukemia Group B Trial 9732. J Clin Oncol 2005;23:3752-9. [PubMed]
- Schiller JH, Adak S, Cella D, et al. Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593-- a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:2114-22. [PubMed]
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85-91. [PubMed]
- Ettinger DS, Jotte R, Lorigan P, et al. Phase II study of amrubicin as second-line therapy in patients with platinum-refractory small-cell lung cancer. J Clin Oncol 2010;28:2598-603. [PubMed]
- Shimokawa T, Shibuya M, Kitamura K, et al. Retrospective analysis of efficacy and safety of amrubicin in refractory and relapsed small-cell lung cancer. Int J Clin Oncol 2009;14:63-9. [PubMed]
- Bogart JA, Herndon JE 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys 2004;59:460-8. [PubMed]
- Rosti G, Bevilacqua G, Bidoli P, et al. Small cell lung cancer. Ann Oncol 2006;17:ii5-10. [PubMed]
- Perry MC, Eaton WL, Propert KJ, et al. Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med 1987;316:912-8. [PubMed]
- Johnson DH, Bass D, Einhorn LH, et al. Combination chemotherapy with or without thoracic radiotherapy in limited-stage small-cell lung cancer: a randomized trial of the Southeastern Cancer Study Group. J Clin Oncol 1993;11:1223-9. [PubMed]
- Osterlind K, Hansen HH, Hansen HS, et al. Chemotherapy versus chemotherapy plus irradiation in limited small cell lung cancer. Results of a controlled trial with 5 years follow-up. Br J Cancer 1986;54:7-17. [PubMed]
- Coy P, Hodson I, Payne DG, et al. The effect of dose of thoracic irradiation on recurrence in patients with limited stage small cell lung cancer. Initial results of a Canadian Multicenter Randomized Trial. Int J Radiat Oncol Biol Phys 1988;14:219-26. [PubMed]
- De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, et al. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol 2006;17:543-52. [PubMed]
- Hochstenbag MM, Twijnstra A, Wilmink JT, et al. Asymptomatic brain metastases (BM) in small cell lung cancer (SCLC): MR-imaging is useful at initial diagnosis. J Neurooncol 2000;48:243-8. [PubMed]
- Le Péchoux C, Arriagada R. Prophylactic cranial irradiation in small cell lung cancer. Hematol Oncol Clin North Am 2004;18:355-72. [PubMed]
- Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357:664-72. [PubMed]
- Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99-01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol 2009;10:467-74. [PubMed]
- Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341:476-84. [PubMed]
- Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:77-84. [PubMed]
- Slotman BJ, Mauer ME, Bottomley A, et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: short-term health-related quality of life and patient reported symptoms: results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J Clin Oncol 2009;27:78-84. [PubMed]
- Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet 1973;2:63-5. [PubMed]
- Martini N, Wittes RE, Hilaris BS, et al. Oat cell carcinoma of the lung. Clin Bull 1975;5:144-8. [PubMed]
- Mountain CF. Clinical biology of small cell carcinoma: relationship to surgical therapy. Semin Oncol 1978;5:272-9. [PubMed]
- Kreisman H, Wolkove N, Quoix E. Small cell lung cancer presenting as a solitary pulmonary nodule. Chest 1992;101:225-31. [PubMed]
- Maranzano E, Trippa F, Casale M, et al. 8Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiother Oncol 2009;93:174-9. [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [PubMed]
- Ferrell B, Koczywas M, Grannis F, et al. Palliative care in lung cancer. Surg Clin North Am 2011;91:403-17. [PubMed]


