Pathophysiology and classification of primary graft dysfunction after lung transplantation
Definition of primary graft dysfunction (PGD)
PGD is a syndrome that encompasses a spectrum of mild to severe lung injury that occurs within the first 72 h after lung transplantation. PGD is a major cause of early morbidity and mortality in lung transplantation and is characterised by progressive hypoxaemia and alveolar infiltrates on a chest radiograph. Following ischaemia in the donor organ and reperfusion in the recipient, inflammatory and immunological injury-repair responses seem to be the key pathological mechanisms. PGD has significant impact on the short and longer-term outcomes for lung transplant patients, however strategies aimed at identifying and reducing PGD risk are being developed. Ex vivo lung perfusion (EVLP) is one strategy to improve assessment of the donor organ and has the potential to act as a platform for implementing interventions to reduce the risk of or prevent PGD. This review will provide an overview of the pathophysiology of PGD and its implications in the clinical setting.
In 2005, the International Society for Heart and Lung Transplantation (ISHLT) published their standardised definition for PGD (updated in 2016) (1). In this definition, the PaO2/FiO2 (P/F) ratio and the presence of bilateral infiltrates on a chest radiograph consistent with non-cardiogenic pulmonary oedema are assessed, see Table 1. Assessment is carried out at specific time points after reperfusion; within the first 6 h (T0), post 24 h (T24), 48 h (T48) and 72 h (T72). Ideally, the P/F ratio is measured on a FiO2 of 1.0 and positive end expiratory pressure (PEEP) of 5 cm H20. The radiographic findings of PGD are non-specific and include peri-hilar ground glass opacities, peri-bronchial, perivascular thickening and reticular interstitial and airspace opacities located in a dependent fashion. The 2016 Consensus Group has clarified that the PGD timing starts at the point of reperfusion following release of the second lung recipient pulmonary arterial cross clamp. The Consensus Group proposes that no changes be made to the grading time points of T0, T24, T48, and T72 and reaffirms this approach and now clarifies that “any” P/F ratio is to be considered grade 0 in the absence of radiographic diffuse pulmonary oedema.

Full table
Other contributory factors that can mimic, modify and confound the definition and grading need to be excluded, including cardiogenic oedema, pneumonia, hyper-acute rejection and pulmonary venous anastomotic obstruction.
Epidemiology and clinical outcomes
Historically it was difficult to accurately assess the incidence of PGD due to varying definitions and even with the introduction of the ISHLT standardised definition in 2005; there is still a dependence on accuracy in the timing and severity grading of PGD. Since the implantation of the standardised ISHLT definition, the incidence of severe PGD grade 3 (PGD3) within 48–72 h postoperatively has been reported at approximately 10–20% and the incidence of PGD3 at any time point within the first 3 days, approximately 30% (2-4). Diamond et al. demonstrated in a large, multi-centre, prospective cohort study of 1,255 lung transplant recipients between 2002 and 2010, that there was an overall incidence of PGD grade 3 of 30.8% at any time point during the first 72 h of lung transplantation, while grade 3 PGD present at 48 or 72 h after reperfusion had an incidence of 16.8% (5).
PGD is associated with significant early and late post-transplant morbidity: patients with PGD3 have longer hospital and intensive care length of stays, duration of mechanical ventilation, increased short and longer-term mortality than those with lower grades of PGD. Whitson et al. analysed 402 lung transplant recipients at the University of Minnesota from 1992 to 2004, reporting that that the 90-day mortality rate associated with the occurrence of PGD grade 3 at any point within the first 48 h after transplantation was 17% versus 9% in the group without grade 3 PGD (6). In a study of the United Network for Organ Sharing (UNOS)/ISHLT database between 1994 and 2000, all-cause mortality at 1-year was 64.9% in recipients with PGD 3 beyond 48 h versus 20.4% in the non-PGD group (7). Diamond et al. showed that PGD was significantly associated with 90-day and 1-year mortality with grade 3 PGD at 48 or 72 h after transplant being associated with an absolute risk increase for death within 90 days compared to those without grade 3 PGD of 18% and 23% for death within 1 year (5). PGD has also been associated with an increased risk of developing bronchiolitis obliterans syndrome (BOS) which is the main limiting factor for long-term survival after lung transplantation (8).
Whitson et al. showed that patients with PGD3 had a significantly reduced longer term survival compared with those PGD1 and PGD2; median survival grade 3: 4.6 years; grade 2: 6.6 years; grade 1: 7.5 years (6). Kreisel et al. reported significant association between PGD with decreased long-term survival in their single centre cohort study of 1,000 recipients. They showed 1-, 5- and 10-year survival to be 72.8%, 43.9% and 18.7% in the PGD group compared with 87.1%, 59.8% and 35.7% in the non-PGD group respectively (9). PGD has also been shown to significantly impact on recipient functional status which ultimately will impact on quality of life. For example, survivors of PGD 3 at 12 months were seen to have a much shorter 6 min walk distance than those without (10).
Risk factors: pre-transplantation
With regard to the overall lung transplant procedure there are several places that can act as potential risk factors for subsequent PGD. Brain death leads to haemodynamic compromise, hormonal derangements, hypothermia and release of inflammatory cytokines (11,12). Warm ischaemia occurring after loss of circulation during organ retrieval and then cold ischaemia to preserve the organs causes a deterioration in tissue oxygenation. A release of inflammatory cytokines, namely IL-8, IL-12, IL-18, TNF-alpha and IFN-gamma triggers a cascade of tissue apoptosis, necrosis and results in organ dysfunction (11). The pathophysiology will be discussed in depth later in this review.
Hypoxia during ischaemic periods promotes coagulation through activation of endothelial cells, leading to a pro-coagulable environment. In an analysis of the UNOS database, an association with PGD was found with donor traumatic brain injury (TBI) as a cause of death (13). Diamond et al., however reported in a large multicentre cohort study that cause of donor death was not a risk factor for PGD (5).
There are increasing numbers of lung transplantations following donation after circulatory confirmation of death (DCD), with differing opinions on the effect of type of donation, DCD versus to donation after brain death (DBD) on PGD risk. A meta-analysis of five studies found no difference in PGD incidence between DCD and DBD donors (14). A study published the same year, however conversely reported that there was a higher incidence of PGD and a trend towards greater need for extracorporeal life support (ECMO) in a series of 60 DCD transplant recipients compared to their score matched DBD recipients (15).
Risk factors: post-transplantation
Donor-related factors
Donor-related factors can be categorised as hereditary and acquired. PGD risk has been reported to increase in donors older than 32–45 years (16). Whitson et al. demonstrated that the relationship between the development of PGD3 and donor age in their series was linear from the age 35 years upwards and a 3% increased risk of PGD per one-year increased in donor age (6). More recent data from Baldwin et al. suggests that the age-related risk of PGD is restricted to the extremes of age and that donor age 55 to 64 years was not associated with a significantly increased risk of severe PGD after controlling for recipient, surgical and other donor factors (17).
Other donor risk factors reported include age, African American race, female gender, and history of tobacco exposure (6,12,13,16,18). Bonser et al. reviewed the effect of donor smoking after lung transplantation using a cohort study of a prospective registry and found that donor smoke exposure was associated with worse recipient outcomes (19). The survival probability, however, still exceeded that of remaining on the waiting list. Alcohol use also appears to increase PGD risk, with donors categorised as “heavy drinkers” displaying a 9-fold higher risk of severe PGD compared to those with no alcohol intake (20).
Potential donor risk-factors that are acquired include prolonged periods of mechanical ventilation, aspiration, pneumonia, excessive blood transfusion and trauma, however further definitive studies are needed to demonstrate their associations with PGD. The presence of fat embolism from the donor is said to increases the risk of PGD development by 25-fold (21).
Recipient-related factors
The primary diagnosis leading to the need for lung transplantation is an important modifier of the risk of developing PGD. The most significant recipient risk factors are obesity (22,23), pulmonary hypertension and diagnoses of pulmonary fibrosis (24) and/or sarcoidosis (6,25). In a systematic review of 10 studies, the incidence of PGD was 11.8% in patients with chronic obstructive pulmonary disease (COPD), 12.4% in cystic fibrosis, 18.0% in patients with idiopathic pulmonary fibrosis (IPF), 50% in sarcoidosis and 30.3% in patients with idiopathic pulmonary arterial hypertension (IPAH) (18). Several studies including a meta-analysis, large multicentre cohort study and a single centre study have shown IPAH was strongly associated with risk for PGD. This remains the case even after adjustment for recipient pulmonary artery systolic pressure, with reports highlighting that higher pulmonary arterial pressure has been shown to be strongly correlated with the development of PGD, with a 30% increased risk of PGD for every 10 mmHg increase in mean PAP (5,16,18). After adjustment for multiple risk factors, obese and overweight recipient body mass index (BMI) were independent predictors of PGD. Lederer et al. demonstrated a link between plasma levels of leptin which is associated with adiposity and risk of PGD with obesity being associated with greater than a 2-fold increased risk of grade 3 PGD within 72 h postoperatively in adult lung transplant recipients with COPD or ILD (22).
Pre-transplantation biological levels of certain markers have also been shown to have an association with a higher risk of PGD development. Cantu and colleagues found that donor polymorphisms in the oxidant stress gene NOX3 were associated with increased risk of PGD (26). Machuca and colleagues found that lungs that subsequently developed PGD had higher levels of IL-8, macrophage colony stimulating factor and growth-related oncogene-α compared to lungs that did not develop PGD (27). More PGD-associated biomarkers will be discussed later on in the review.
Operative/surgical factors
The operative risk factors for PGD reported are single lung transplant procedure, prolonged ischaemia time, cardiopulmonary bypass (CPB) use, red blood cell transfusion greater than 1 litre and reperfusion FiO2 greater than 0.4 (5,13,28-30). Weber et al. reported that patients who received an intraoperative blood transfusion of greater than 4 units were seen to have an increase in PGD, renal replacement therapy, a post-operative ECMO requirement and mortality (31). CPB has been associated with an increased risk of PGD, reported by Aeba et al. in a retrospective study over a 2-year period in 100 lung transplant recipients. They found that the cohort of patients whom had CPB had a more prolonged period of intubation and more severe pulmonary infiltrates on radiography (32). Diamond et al. demonstrated in their large, multi-centre cohort study and meta-analysis that CPB was an independent risk factor for the development of PGD (5) and also reported the association between PGD and single lung transplantation. In a meta-analysis, however this did not seem to be the case (18).
Reperfusion FiO2 was also seen to be an independent risk factor for PGD in a large, 10-centre prospective cohort study with an increased risk of PGD at 48 or 72 h reported in patients where the reperfusion FiO2 had been >0.4, with an absolute risk increase of 6% when compared to a reperfusion FiO2 of <0.4 (5).
Pathophysiology of primary graft dysfunction
The underlying causes of PGD remain poorly defined. However, the emergence of data from large, multicentre-derived lung transplant recipient outcome studies and genetic data sets has allowed for the development in the understanding of the PGD mechanisms at a cellular and molecular level.
Neutrophil activation and interactions with the endothelium
The hallmark of PGD pathophysiology is the migration of polymorphonuclear neutrophils (PMNs) from the pulmonary circulation into interstitium and the airways. These PMNs are attracted out of the circulation by chemotactic mediators such as CXCL8 and damage-associated molecular patterns (DAMPs) which have been released from apoptotic and necrotic lung tissue following ischaemia-reperfusion injury (IRI). This leads to the release of DAMPs into the circulation, with HMGB1 and ATP being well-known examples. A hyperoxic mouse model of acute lung injury (ALI) utilised by Entezari et al. identified high levels of HMGB1 in the bronchoalveolar lavage fluid (BALF) of these mice (33). Intra-tracheal administration of additional HMGB1 within this model served to further exacerbate the symptoms of ALI in this model by mediating leukocyte infiltration into the airways. Shah et al. used another model of pulmonary inflammation to identify extracellular ATP as another potent mediator of neutrophil recruitment into the lungs during ALI (34). Both HMGB1 and ATP are well-renowned DAMPs, thus IRI-mediated damage of the pulmonary airways serves to release these into the circulation and facilitate attraction of neutrophils to the lungs in the case of acute pulmonary diseases such as PGD.
Alongside neutrophil activation and migration, activation of pulmonary endothelium is another very important part of the pathophysiology of PGD. This activation occurs through the release of pro-inflammatory cytokines TNFα and IL-1β which enables up-regulation of adhesion molecules on the surface of the endothelium (35,36). E-selectin on the endothelial surface interacts with P- and L-selectin found on neutrophils to facilitate weak binding, as the latter roll along the endothelial surface through a cytokine ‘gradient’ before being arrested via integrin binding. Mac-1 and α4β1 have been shown to play essential roles in recruiting neutrophils to the lungs during S. Pneumoniae infection and thus likely enable this process in the case of inflammation produced in PGD (37). Once localised to the lungs, transmigration of PMNs into the interstitial space and airways is facilitated by up-regulation of adhesion molecules, notably ICAM-1 and PECAM-1 (36). Support for the significance of the models is demonstrated by the study conducted by Simms et al., who identified heightened CD11b/CD18 cell surface expression as being associated with individuals who displayed acute respiratory distress syndrome (ARDS) (38). Finally, a study that utilised atopic asthma patients highlighted the fact that a CXCR1/2 agonist was able to down-regulate the amount of CXCL8-mediated neutrophil infiltration of the airways, indicating that this is a key pathway by which neutrophil trafficking in the context of inflammatory lung disease is enabled (39). Thus, the combination of in vivo models of neutrophil trafficking along with epidemiological studies of associated molecular entities highlight the enormous significance of this process in enabling heightened pulmonary inflammation.
Endothelial cells up-regulate adhesion markers and secrete cytokines in response to IRI, resulting in leukocyte recruitment out of the circulation. The act of interrupting blood flow before restoration is considered the major event in IRI that disrupts the homeostasis of the endothelium. This is a well-documented mechanism in which the sudden re-establishment of blood flow within the organ causes dramatic cell depolarization. A 2011 study of 126 PGD patients between the year 2002 and 2007 was carried out by Fang et al. that investigated the effect of pulmonary arterial pressure on the likelihood of developing grade 3 PGD. The authors found a statistically significant correlation between the two, indicating the effect of significant reperfusion force on endothelial integrity (24). Schnickel et al. analyzed the effects of a modified reperfusion technique involving the insertion of a catheter into the main or individual pulmonary artery after implantation on the incidence of PGD (40). The recipient blood was depleted of leukocytes; supplemented with nitroglycerin; adjusted for pH and calcium level; enriched with aspartate, glutamate, and dextrose; then administered into the pulmonary arteries of the newly transplanted lung(s) for the first 10 min of reperfusion. Severe primary graft dysfunction was defined as a PaO2/inspired oxygen fraction of less than 150 with diffuse infiltrate on the radiograph in absence of other causes. One hundred patients underwent lung transplantation with the modified reperfusion technique. Forty-two patients underwent single-lung transplantation, of which 5 patients required CPB for the procedure. Fifty-eight patients underwent double-lung transplantation; all double-lung transplantation procedures were performed with patients on CPB. There were no technical complications associated with the modified reperfusion, with the mean PaO2/inspired oxygen fraction at 6 h in this cohort being 252±123 mmHg. The median number of days on the ventilator was 2, with incidence of severe primary graft dysfunction in this cohort being 2.0%. The early survival (30-day or in-hospital mortality) of this group of patients was 97%. They concluded that the technique of modified reperfusion in human lung transplantation is associated with a low incidence of severe primary graft dysfunction and favorable short-term outcomes. Another study by Porteous et al. highlights that diastolic dysfunction also plays a role in increasing the risk of PGD development (41). These studies provide substantial evidence for the key role that flow along the endothelium has in instigating lung damage, as IRI is essential in enabling PGD to develop.
Initiation of IRI provokes endothelial cells to up-regulate various adhesion markers on their surface, produce cytokines and begin actively contributing to inflammation (42-44). ICAM-1 and PECAM-1 have both been implicated in ALI and inflammation in general, with these facilitating leukocyte transmigration from the circulation into the airways (35). ICAM-1 levels can be seen to relate to patient outcome in human studies (45). The integrins Mac-1 and α4β1 were shown in a mouse model of lung infection by Kadioglu et al. to not only be essential in allowing neutrophil and T lymphocyte movement into the airways, but also facilitated the immunological response that was directed against the pathogen by these cells (37).
The other major effect that stimulation of the endothelium causes is production of cytokines by these cells. Endothelial cells have been shown to directly participate in amplifying the inflammatory response and can produce PGD-associated cytokines including PAI-1 and IL-17. These are detrimental to the function of the lung, with levels of both of these examples having been linked with worsening outcomes of PGD in patients (46-48). Activation of endothelial cells primes the environment for the secondary ‘wave’ of leukocyte influx into the pulmonary airways. It is this secondary phase that is ultimately so damaging.
Many key mediators released as a result of inflammation can also directly affect the structural integrity and viability of the endothelium. IRI initiates generation of caspase-8 and -9 that have been shown in a rat model to sustain mitochondrial injury and overall contribute in a detrimental manner to the consequences of reperfusion. In lung injury, apoptosis has generally been shown to play a key role in mediating significant tissue damage (49,50). Li et al. highlight how TNF-alpha and LPS-mediated damage in vitro triggers activation of MAPK pathways that culminates in substantially increased vascular endothelial permeability, translating to impaired barrier functions within an in vivo setting and disrupting gaseous exchange within the airways. CXCL8, which is released in large quantities by a variety of cells to attract leukocytes out of circulation has been shown in vitro to directly regulate cell permeability by causing down-regulation of tight junctions. This was seen to operate in a time-dependent manner, putting forward the likely scenario that accumulated CXCL8 gradually wears down the endothelial integrity over time (51). This is in turn supported by human studies which have shown that IL-8 levels in the lungs of DBD donors correlate in a concentration-dependent manner to PGD severity and mortality (52). Vascular permeability is therefore one of the major facets in lung insult and thus plays a large role in PGD pathogenesis.
Once localised to the airways, neutrophil activation contributes to the significant inflammation in this microenvironment. Multiple studies highlight how platelet-neutrophil interactions are key to enabling PMN activation in a number of autoimmune disease pathologies, including acute lung conditions (53-55). This subsequent activation is associated with production of numerous protease enzymes. Neutrophil elastase (NE) can utilise a wide range of extracellular matrix (ECM) proteins as substrates and thus disrupting airways integrity. Furthermore; release of cytokines such as IL-1β have also been attributed to the action of this enzyme. A study by Ishii et al. used a bilateral nephrectomy model to induce ALI and identified that application of an NE antagonist reduced levels of cytokines such as IL-6, as well as numerous other inflammatory processes associated with this model (56). Matrix metalloproteinases (MMPs) target a wide range of substrates, with neutrophils being known to secrete these when activated. MMP-8 (neutrophil collagenase) levels in BALF were correlated with severity of disease in bronchiectasis patients, whilst another study found that blood levels of MMP-9 could be used to help differentiate between PGD grades 2/3 and individuals with no PGD present (57). These studies strongly support neutrophil degranulation being a major contributory factor to PGD disease progression.
Neutrophils are also responsible for the secretion of neutrophil extracellular traps (NETs) (58). These are strands of chromatin with the ability to bind a range of intercellular secretions and granules and ultimately ensnare extracellular pathogens. Sayah et al. used two experimental murine models to confirm that the presence of platelet-mediated NET formation contributed to PGD pathology. Prevention of NET formation by direct disruption with DNase I was seen to reduce the amount of lung injury present. Epidemiological evidence for this pathogenic role is provided in the study, as human BALF samples were analysed for the presence of NE-DNA complexes. Generally, these were present to a much higher degree within individuals with moderate to severe grades of PGD (59). Table 2 outlines the enzymes secreted by activated neutrophils and the role that plays in PGD pathophysiology.
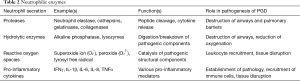
Full table
Alveolar macrophages (AMs) activation and leukocyte recruitment
AMs are a niche population that are located within the alveolar space and airways and are continuously surveying the airways for pathogen-associated molecular patterns (PAMPs). The pro-inflammatory functions of AMs are normally tightly regulated by epithelial signals, including CD200 binding and secretions of the cytokines TGF-β and IL-10 (60). Damage to the airways caused as a result of IRI provokes an initial wave of cellular apoptosis, followed by a subsequent secondary necrosis. This releases an array of previously detailed DAMPs such as RAGE and HMGB1, with the latter in particular stimulating AMs to migrate to the site of inflammation (61). Damage to the epithelium also has the effect of decreasing the concentrations of anti-inflammatory mediators that are continuously secreted into the airways. Lack of effective cellular regulation produces populations of cells with an inflammatory profile, characterised in the case of kidney IRI by heightened cytokine production and increased oxidative burst that resembles an ‘M1’ polarized phenotype (62,63). Several studies have highlighted the importance of AMs in establishing IRI (64-66). A study carried out in rat lungs utilised liposomal clodronate to knockdown resident macrophage populations. This not only reduced the concentration of several known inflammatory cytokines, including; MIP-1, MIP-2α and TNFα, but also protected against much of the epithelial damage that is such a hallmark of IRI (67). Balamayooran et al. used an MCP-1-/- mouse strain to demonstrate that these mice had a significantly impaired clearance of bacteria when infected with Escherichia coli, caused by lower cytokine levels, less cellular adhesion and a reduced capacity of neutrophil influx. Shah et al. correlate this in patients, noting that levels of MCP-1 at 24 h can be used as a biomarker for PGD (45,68). Other models of lung diseases such as IPF also display a similar dependency on AMs to initiate inflammation (66). Many of these studies further demonstrate that depletion of this niche macrophage population can often alleviate much of the damage observed in these attempts to model ALI. Finally, the evidence gathered by these models is supported by in vitro data, as AMs have been shown to produce levels of the pro-inflammatory cytokines IL-1β, IL-6, IL-8 and TNFα when stimulated, demonstrating directly relatable proof for these mechanisms of action (69,70).
Disruption of the tissues - loss of epithelial integrity
Multiple studies identifying epithelial injury markers having a high association with more severe grades of PGD. Patel et al. note the detrimental effect that TNF-mediated cell death has on epithelial integrity in their 2013 study, with the presence of TNFα already being well established in the pathology of ALI in general with regard to leukocyte recruitment into the airways (71). RAGE is a major DAMP in PGD and levels have been correlated with higher grades of disease and linked to lengthened hospital stays by multiple patient studies, underlining how disruption of epithelial integrity appears to be a major factor in post-transplant failure (34,72). Expression levels were found to correlate with increased length of hospitalization by Pelaez et al. in a 2010 study of PGD patient BALF samples (73). RAGE is of course known to be a receptor for the aforementioned DAMP HMGB1, thus higher levels of RAGE on the surface of leukocytes enables higher levels of activation through this pathway and thus worsens the pathology of PGD. This link is confirmed in a model by Sharma et al. (2013) who used an in vivo hilar clamp model to induce IRI in C57BL/6 wild-type mice. Upon treatment with recombinant HMGB1, symptoms of IRI considerably worsened, but this was not the case when the same model was applied to RAGE-/- mice (74).
One notable contributory factor towards PGD is the presence of pre-transplant anti-col(V) antibodies. Iwata et al. identified in a 2008 study that epithelial tissue and not endothelial tissue showed expression of this, which explained why exclusively epithelial cells were targeted by an anti-col(V), CD4+ mediated cytotoxic response. Examination of patient samples identified that individuals with pre-formed antibodies had strong likeliness of developing PGD, confirming this link (75). This autoantibody response has been confirmed as playing a significant role in a murine model by Bharat et al. Here, the authors note that in a syngeneic transplantation model, lung-restricted antibodies directed against col(V) showed a dose-dependent response, characterised by poor oxygenation compared to the isotype controls performed (76). Pre-formed anti-col(V) antibodies in patients are therefore a significant factor in potentially damaging the epithelium in acute lung injuries.
Whilst epithelial damage is indeed an early hallmark of primary graft dysfunction evidence supports the contributory role that these cells in fact have towards worsening of pathology in many cases. One study highlighted that once produced; IL-17 and TNFα act in synergy with one another to promote the epithelial up-regulation of CXCL1, which mediates potent migration of neutrophils to the site of expression, as well as being known to modulate leukocyte functions in immunity (47,48,77). This observation was also correlated with significantly less pulmonary dysfunction when this NADPH-mediated pathway was blocked in vivo in a study by Sharma et al., who noted down-regulation in expression of CXCL1, as well as CCL2, CCL5 and IL-6 (78). The epithelial layers therefore are not only significant targets in IRI, but also actively support the progression of this injury to development of clinically relevant PGD.
Recent studies have started to map out the signalling pathways that lead to IRI. One such pathway involves toll-like receptors (TLRs); an important class of pattern-recognition receptor that are located in abundance on the surface of AMs. Several studies have attempted to model ALI and highlighted the importance of TLR4 in establishing ALI pathology. A study in 2012 utilised pulmonary arterial occlusion to highlight that mice could generate inflammatory mediators when exposed to a model of IRI, with this capability being abrogated if TLR4-/- mice were used (79). One in vivo model finding that using TLR4-/- mice was sufficient to not only reduce the amount of pro-inflammatory markers produced in the wild-type, but also prevented efficient neutrophil recruitment into the airways (79). Ding et al. also managed to identify that activated TLR4 can bring about activation of TLR3 via an NF-κβ-mediated pathway and that this leads to an up-regulation of PMN migration in the context of ALI (80). Further evidence for the importance of the role of TLR4 is demonstrated by Xu et al. who note that levels of micro RNA-21 (miR-21) have been noted as being reduced in patients with higher grades of PGD. This microRNA acts to down-regulate TLR4 expression, as well as levels of other signalling molecules, highlighting a pathway by which individuals with lower levels may be more susceptible to excess inflammation post-transplantation (81). AM populations by Dhaliwal et al. were seen to reduce neutrophil influx into the airways (67,79). The authors note that once this damage has been avoided, replenishment of macrophage populations is not sufficient to initiate damage to the airways, but this can be done via later administration of PMNs, effectively signalling the ‘guiding’ role that AMs perform within PGD and other pulmonary abnormalities. This provides evidence for the importance of AMs in modulating PMN migration in the context of ALI and subsequent development into severe grades of PGD. Lastly, TLR4 has been further implicated in PGD progression by activating NETosis in neutrophils, which also correlates with PGD grade. Knockdown of macrophages also prevented IRI development in this model, with lack of neutrophil infiltration into the airways remaining absent even after 3 h had elapsed (53). NETosis will be discussed in more depth later.
The role of CD4+ T lymphocytes in facilitating leukocyte recruitment
Lymphocytes are typically more involved in the latter stages of PGD development. Once the ‘resident’ AMs have detected and responded to IRI within the lungs, they can then initiate intercellular communication with donor T lymphocytes. CD4+ cells mediate these effects in PGD, and to a much lesser extent CD8+ cells. In a murine model of IRI; depletion of T lymphocytes was seen to be sufficient to alleviate the worst of the pathologically associated symptoms (82). The authors also note that depletion of CD4+ T lymphocytes within this system was sufficient to reduce the amount of PMN infiltration into the tissues. Evidence therefore points towards them having a ‘chaperone’ role; in that they stimulate and direct neutrophils to infiltrate the lung, which will then cause the majority of the damage to the pulmonary environment. The mechanisms of infiltration into the airways appear to be homologous with those utilised by PMNs in the case of lung disease. A combination of Th2 and Th17 responses have been identified in PGD and are synonymous with severity of disease in both cases. Levels of the cytokines IL-23 and especially IL-17 have been heavily linked with development of PGD pathology (83).
As highlighted in the section on epithelial damage; pre-formed anti col(V) antibodies are a significant factor in the outcome of a lung transplant, with immunity mediated by CD4+ T cells being identified by many studies as a significant observation within PGD. Circulating antibodies bind to these regions on epithelial cells and the exposed Fc regions will then bind to receptors on the surface of professional phagocytes, namely the AMs present in the airways. Numerous studies have documented the presence of anti-col(V) immunity present within PGD patients (75,84). This is further corroborated by the links between antibody presence and transplant outcome which again point towards a higher incidence being of great significance in development of graft injury.
Mechanisms involved in PGD progression and disease-associated biomarkers
Much of the work to identify biomarkers of PGD has come from the ‘Lung Transplant Outcomes Group’. This was sponsored by the United States National Institutes of Health and collected data on lung transplant patients at 10 transplant U.S. centres between 2002 and 2010. Links have been made between relative levels of the proteins listed in Table 3 and risk of PGD development. Whilst much of this information on molecular associations with outcomes in PGD remains epidemiological, recent studies have begun to attempt to map out many of the signalling pathways that lead to exacerbation of IRI. Implicated pathways within the pathogenesis involve receptors such as the toll- and NOD-like receptors (TLRs and NLRs) and up-regulation of cytokines such as IL-1β. Continued research into these pathways remains ongoing in the hope of identifying novel targets for therapeutic intervention.
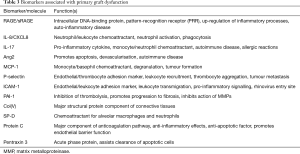
Full table
Shah et al. measured levels of a panel of biomarkers in the plasma of a cohort of 315 PGD patients. This contained molecules involved in a range of processes such as lung epithelial damage (sRAGE), cellular adhesion (ICAM-1) and coagulation (PAI-1). All were successfully linked with PGD grade and when used in combination with one of the others and the authors conclude that addition of any one of these to the clinical PGD grading significantly improved 90-day mortality prediction (85). Another detailed analysis of patient BAL fluid samples by Cantu et al. in 2013 revealed a wide range of immune pathways that associated heavily with enhanced cytokine release in the case of PGD development. IL-1, IL-6 and CCL4 all showed enhanced expression levels, with signalling pathways associated with immune receptors such as the TLRs were all expressed to higher degrees as well (26).
These aforementioned receptors become activated to release substantial amounts of pro-inflammatory mediators. Notable examples in the case of acute lung pathologies are CXCL8 and IL-17. With regard to the former, a 2001 study by Fisher et al. highlighted how levels of IL-8 within donor lungs could be linked to graft failure early on post-transplantation (86). As previously detailed, this cytokine plays a key role in recruiting PMNs and other leukocytes into the pulmonary airways during inflammation (87). The indispensable role of IL-17 within auto-inflammatory diseases is well-renowned and has been closely linked to PGD progression. IL-17 receptor (IL-17R) polymorphism has also been shown to display a predisposition to PGD development post-lung transplantation, suggesting a role for assisting neutrophil recruitment to the lungs (47).
Prevention and treatment of PGD
Due to a lack of appropriately powered clinical studies, there is no clear, overall consensus on the treatment of PGD after lung transplantation. Given the similar clinical features and radiographic findings seen in both PGD and ARDS, many of the potential treatment options have been extrapolated from the management of ARDS. Strategies used to prevent and minimise the development and severity of PGD include optimising donor selection, matching and management pre-operatively; improving lung preservation and storage techniques and improving reperfusion techniques. Several therapeutic agents have been studied in an effort to reduce the incidence of PGD, with a selection of these being depicted in Table 4. Soluble complement receptor 1 inhibitor, plasminogen activating factor antagonist and exogenous surfactant demonstrated beneficial effects on surrogates of PGD including the A-a gradient.
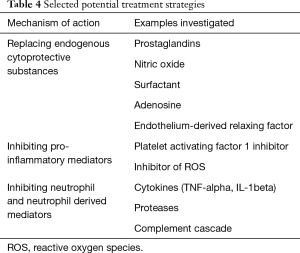
Full table
Surfactant is synthesised by type 2 pneumocytes and secreted into the alveolar space where it forms a stable monolayer, resulting in reduced surface tension in the alveoli, preventing atelectasis and alveolar oedema. Experimental studies have demonstrated the link between ischaemia, cold storage and reperfusion with alterations in surfactant composition and function leading to reduction in pulmonary compliance, atelectasis, pulmonary oedema and oxygenation (88). Amital et al. in a prospective randomised study demonstrated that delivery of surfactant through a bronchoscope after bronchial anastomosis is established could improve oxygenation and lead to reduced PGD grade, severe PGD rates, earlier extubation and shorter ICU length of stay (89).
Recommendations have emerged for lung protective ventilation (LPV) for PGD patients based on the pre-existing evidence for this strategy in ARDS.
Veno-arterial ECMO has been used for salvage of refractory hypoxaemia due to severe PGD following lung transplantation. VA-ECMO intraoperatively instead of CPB has been associated with shorter duration of mechanical ventilation and ICU/hospital length of stay, and lower transfusion requirements, but no statistically significant difference in 90-day mortality (90).
Normothermic EVLP is an emerging evaluation technique for high risk donor organs and allows for a period of normothermic assessment and reconditioning (47,91-96). Steen et al. in Lund, Sweden were the first to successful carry out EVLP in 2001 using DCD donor lungs. Cypel and colleagues then published results of their landmark study in 2011 where they reported excellent clinical outcomes using donors considered unsuitable for transplantation (97). EVLP also offers a platform through which to deliver targeted therapeutic agents to enhance the quality of the donor lungs. The results of a randomised control clinical trial using a portable extracorporeal perfusion system with the aim to reduce ischaemic time are awaited (98,99).
Conclusions
PGD has significant impact on the short and longer-term outcomes for lung transplant patients, and understanding its underlying pathophysiology is crucial to developing novel strategies aimed at identifying and reducing PGD risk. The immunological and inflammatory cellular and molecular mechanisms involved in the pathogenesis of PGD are highly complex and future experimental models are needed to investigate these further. Findings from PGD patient multicentre outcome and genetic studies are now being analysed in experimental lung transplant models with the aim to develop new therapeutic approaches.
Acknowledgements
The work has been supported by funding to Morvern Morrison and Thomas Pither from the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed of those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health or the NHSBT.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Snell GI, Yusen R, Weill D, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part I: Definition and Grading A 2016 consensus group statement of The International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Christie JD, Bellamy S, Ware LB, et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2010;29:1231-9. [Crossref] [PubMed]
- Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2007;175:507-13. [Crossref] [PubMed]
- Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004-11. [Crossref] [PubMed]
- Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. [Crossref] [PubMed]
- Whitson BA, Nath DS, Johnson AC, et al. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg 2006;131:73-80. [Crossref] [PubMed]
- Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med 2005;171:1312-6. [Crossref] [PubMed]
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31:1073-86. [Crossref] [PubMed]
- Kreisel D, Krupnick AS, Puri V, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg 2011;141:215-22. [Crossref] [PubMed]
- Prekker ME, Nath DS, Walker AR, et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2006;25:371-8. [Crossref] [PubMed]
- de Perrot M, Liu M, Waddell TK, et al. Ischemia–Reperfusion–induced Lung Injury. Am J Respir Crit Care Med 2003;167:490-511. [Crossref] [PubMed]
- Gelman AE, Fisher AJ, Huang HJ, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part III: Mechanisms: A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kuntz CL, Hadjiliadis D, Ahya VN, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant 2009;23:819-30. [Crossref] [PubMed]
- Krutsinger D, Reed RM, Blevins A, et al. Lung transplantation from donation after cardiocirculatory death: a systematic review and meta-analysis. J Heart Lung Transplant 2015;34:675-84. [Crossref] [PubMed]
- Sabashnikov A, Patil NP, Popov AF, et al. Long-term results after lung transplantation using organsfrom circulatory death donors: a propensity score-matched analysis. Eur J Cardiothorac Surg 2016;49:46-53. [Crossref] [PubMed]
- Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest 2003;124:1232-41. [Crossref] [PubMed]
- Baldwin MR, Peterson ER, Easthausen I, et al. Donor age and early graft failure after lung transplantation: a cohort study. Am J Transplant 2013;13:2685-95. [Crossref] [PubMed]
- Liu Y, Liu Y, Su L, et al. Recipient-related clinical risk factors for primary graft dysfunction after lung transplantation: a systematic review and meta-analysis. PLoS One 2014;9:e92773. [Crossref] [PubMed]
- Bonser RS, Taylor R, Collett D, et al. Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet 2012;380:747-55. [Crossref] [PubMed]
- Lowery EM, Kuhlmann EA, Mahoney EL, et al. Heavy alcohol use in lung donors increases the risk of primary graft dysfunction. Alcohol Clin Exp Res 2014;38:2853-61. [Crossref] [PubMed]
- Oto T, Excell L, Griffiths AP, et al. The implications of pulmonary embolism in a multiorgan donor for subsequent pulmonary, renal, and cardiac transplantation. J Heart Lung Transplant 2008;27:78-85. [Crossref] [PubMed]
- Lederer DJ, Kawut SM, Wickersham N, et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med 2011;184:1055-61. [Crossref] [PubMed]
- Lederer DJ, Wilt JS, D'Ovidio F, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med 2009;180:887-95. [Crossref] [PubMed]
- Fang A, Studer S, Kawut SM, et al. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest 2011;139:782-7. [Crossref] [PubMed]
- Barr ML, Kawut SM, Whelan TP, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part IV: recipient-related risk factors and markers. J Heart Lung Transplant 2005;24:1468-82. [Crossref] [PubMed]
- Cantu E, Lederer DJ, Meyer K, et al. Gene set enrichment analysis identifies key innate immune pathways in primary graft dysfunction after lung transplantation. Am J Transplant 2013;13:1898-904. [Crossref] [PubMed]
- Machuca TN, Cypel M, Yeung JC, et al. Protein expression profiling predicts graft performance in clinical ex vivo lung perfusion. Ann Surg 2015;261:591-7. [Crossref] [PubMed]
- Nagendran M, Maruthappu M, Sugand K. Should double lung transplant be performed with or without cardiopulmonary bypass? Interact Cardiovasc Thorac Surg 2011;12:799-804. [Crossref] [PubMed]
- Szeto WY, Kreisel D, Karakousis GC, et al. Cardiopulmonary bypass for bilateral sequential lung transplantation in patients with chronic obstructive pulmonary disease without adverse effect on lung function or clinical outcome. J Thorac Cardiovasc Surg 2002;124:241-9. [Crossref] [PubMed]
- Thabut G, Vinatier I, Stern JB, et al. Primary graft failure following lung transplantation: predictive factors of mortality. Chest 2002;121:1876-82. [Crossref] [PubMed]
- Weber D, Cottini SR, Locher P, et al. Association of intraoperative transfusion of blood products with mortality in lung transplant recipients. Perioper Med (Lond) 2013;2:20. [Crossref] [PubMed]
- Aeba R, Griffith BP, Kormos RL, et al. Effect of cardiopulmonary bypass on early graft dysfunction in clinical lung transplantation. Ann Thorac Surg 1994;57:715-22. [Crossref] [PubMed]
- Entezari M, Javdan M, Antoine DJ, et al. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol 2014;2:314-22. [Crossref] [PubMed]
- Shah D, Romero F, Stafstrom W, et al. Extracellular ATP mediates the late phase of neutrophil recruitment to the lung in murine models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2014;306:L152-61. [Crossref] [PubMed]
- Liu G, Place AT, Chen Z, et al. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood 2012;120:1942-52. [Crossref] [PubMed]
- Liu G, Vogel SM, Gao X, et al. Src phosphorylation of endothelial cell surface intercellular adhesion molecule-1 mediates neutrophil adhesion and contributes to the mechanism of lung inflammation. Arterioscler Thromb Vasc Biol 2011;31:1342-50. [Crossref] [PubMed]
- Kadioglu A, De Filippo K, Bangert M, et al. The integrins Mac-1 and alpha4beta1 perform crucial roles in neutrophil and T cell recruitment to lungs during Streptococcus pneumoniae infection. J Immunol 2011;186:5907-15. [Crossref] [PubMed]
- Simms HH, D'Amico R. Increased PMN CD11b/CD18 expression following post-traumatic ARDS. J Surg Res 1991;50:362-7. [Crossref] [PubMed]
- Todd CM, Salter BM, Murphy DM, et al. The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects. Pulm Pharmacol Ther 2016;41:34-9. [Crossref] [PubMed]
- Schnickel GT, Ross DJ, Beygui R, et al. Modified reperfusion in clinical lung transplantation: the results of 100 consecutive cases. J Thorac Cardiovasc Surg 2006;131:218-23. [Crossref] [PubMed]
- Porteous MK, Ky B, Kirkpatrick JN, et al. Diastolic Dysfunction Increases the Risk of Primary Graft Dysfunction after Lung Transplant. Am J Respir Crit Care Med 2016;193:1392-400. [Crossref] [PubMed]
- Nomellini V, Brubaker AL, Mahbub S, et al. Dysregulation of neutrophil CXCR2 and pulmonary endothelial icam-1 promotes age-related pulmonary inflammation. Aging Dis 2012;3:234-47. [PubMed]
- Young MR. Endothelial cells in the eyes of an immunologist. Cancer Immunol Immunother 2012;61:1609-16. [Crossref] [PubMed]
- Zhang Y, Guan L, Yu J, et al. Pulmonary endothelial activation caused by extracellular histones contributes to neutrophil activation in acute respiratory distress syndrome. Respir Res 2016;17:155. [Crossref] [PubMed]
- Shah RJ, Diamond JM, Lederer DJ, et al. Plasma Monocyte Chemotactic Protein-1 (MCP-1) Levels at 24 hours are a Biomarker of Primary Graft Dysfunction Following Lung Transplantation. Transl Res 2012;160:435-42. [Crossref] [PubMed]
- Sapru A, Curley MA, Brady S, et al. Elevated PAI-1 is associated with poor clinical outcomes in pediatric patients with acute lung injury. Intensive Care Med 2010;36:157-63. [Crossref] [PubMed]
- Somers J, Ruttens D, Verleden SE, et al. Interleukin-17 receptor polymorphism predisposes to primary graft dysfunction after lung transplantation. J Heart Lung Transplant 2015;34:941-9. [Crossref] [PubMed]
- Song HW, Liu W, Yang C, et al. Interleukin-17A promotes paraquat-induced acute lung injury on mice. Int J Clin Exp Pathol 2017;10:2436-45.
- Scarabelli TM, Stephanou A, Pasini E, et al. Different signaling pathways induce apoptosis in endothelial cells and cardiac myocytes during ischemia/reperfusion injury. Circ Res 2002;90:745-8. [Crossref] [PubMed]
- Schmidt EP, Tuder RM. Role of Apoptosis in Amplifying Inflammatory Responses in Lung Diseases. J Cell Death 2010;10:41-53.
- Yu H, Huang X, Ma Y, et al. Interleukin-8 Regulates Endothelial Permeability by Down-regulation of Tight Junction but not Dependent on Integrins Induced Focal Adhesions. Int J Biol Sci 2013;9:966-79. [Crossref] [PubMed]
- De Perrot M, Sekine Y, Fischer S, et al. Interleukin-8 Release during Early Reperfusion Predicts Graft Function in Human Lung Transplantation. Am J Respir Crit Care Med 2002;165:211-5. [Crossref] [PubMed]
- Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 2012;122:2661-71. [Crossref] [PubMed]
- Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007;13:463-9. [Crossref] [PubMed]
- Kornerup KN, Salmon GP, Pitchford SC, et al. Circulating platelet-neutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol (1985) 2010;109:758-67. [PubMed]
- Ishii T, Doi K, Okamoto K, et al. Neutrophil elastase contributes to acute lung injury induced by bilateral nephrectomy. Am J Pathol 2010;177:1665-73. [Crossref] [PubMed]
- Craig VJ, Quintero PA, Fyfe SE, et al. Profibrotic activities for matrix metalloproteinase-8 during bleomycin-mediated lung injury. J Immunol 2013;190:4283-96. [Crossref] [PubMed]
- Jiang S, Park DW, Tadie JM, et al. Human resistin promotes neutrophil pro-inflammatory activation, neutrophil extracellular trap formation, and increases severity of acute lung injury. J Immunol 2014;192:4795-803. [Crossref] [PubMed]
- Sayah DM, Mallavia B, Liu F, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2015;191:455-63. [Crossref] [PubMed]
- Meuth SG, Simon OJ, Gnimm A, et al. CNS inflammation and neuronal degeneration is aggravated by impaired CD200-CD200R-mediated macrophage silencing. J Neuroimmunol 2008;194:62-9. [Crossref] [PubMed]
- Kokkola R, Andersson A, Mullins G, et al. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 2005;61:1-9. [Crossref] [PubMed]
- Huen SC, Huynh L, Marlier A, et al. GM-CSF Promotes Macrophage Alternative Activation after Renal Ischemia/Reperfusion Injury. J Am Soc Nephrol 2015;26:1334-45. [Crossref] [PubMed]
- Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 2011;22:317-26. [Crossref] [PubMed]
- Tatham KC, O'Dea KP, Romano R, et al. Retention and Activation of Donor Vascular Monocytes in Transplanted Lungs Suggests a Central Role in Primary Graft Dysfunction. Am J Respir Crit Care Med 2016;193:A4890.
- Tsushima Y, Jang JH, Yamada Y, et al. The depletion of donor macrophages reduces ischaemia-reperfusion injury after mouse lung transplantation. Eur J Cardiothorac Surg 2014;45:703-9. [Crossref] [PubMed]
- Zhang-Hoover J, Sutton A, Van Rooijen N, et al. A critical role for alveolar macrophages in elicitation of pulmonary immune fibrosis. Immunology 2000;101:501-11. [Crossref] [PubMed]
- Dhaliwal K, Scholefield E, Ferenbach D, et al. Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am J Respir Crit Care Med 2012;186:514-24. [Crossref] [PubMed]
- Balamayooran G, Batra S, Balamayooran T, et al. Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection. Infect Immun 2011;79:2567-77. [Crossref] [PubMed]
- Ishii Y, Yang H, Sakamoto T, et al. Rat alveolar macrophage cytokine production and regulation of neutrophil recruitment following acute ozone exposure. Toxicol Appl Pharmacol 1997;147:214-23. [Crossref] [PubMed]
- Losa García JE, Rodríguez FM, Martín de Cabo MR, et al. Evaluation of inflammatory cytokine secretion by human alveolar macrophages. Mediators Inflamm 1999;8:43-51. [Crossref] [PubMed]
- Patel BV, Wilson MR, O'Dea KP, et al. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol 2013;190:4274-82. [Crossref] [PubMed]
- Scheiber-Camoretti R, Mehrotra A, Yan L, et al. Elevated S100A12 and sRAGE are associated with increased length of hospitalization after non-urgent coronary artery bypass grafting surgery. Am J Cardiovasc Dis 2013;3:85-90. [PubMed]
- Pelaez A, Force SD, Gal AA, et al. Receptor for Advanced Glycation End Products in Donor Lungs Is Associated with Primary Graft Dysfunction After Lung Transplantation. American Journal of Transplantation 2010;10:900-7. [Crossref] [PubMed]
- Sharma AK, LaPar DJ, Stone ML, et al. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury. Am J Transplant 2013;13:2255-67. [Crossref] [PubMed]
- Iwata T, Philipovskiy A, Fisher AJ, et al. Anti-type V collagen humoral immunity in lung transplant primary graft dysfunction. J Immunol 2008;181:5738-47. [Crossref] [PubMed]
- Bharat A, Chiu S, Zheng Z, et al. Lung-Restricted Antibodies Mediate Primary Graft Dysfunction and Prevent Allotolerance after Murine Lung Transplantation. Am J Respir Cell Mol Biol 2016;55:532-41. [Crossref] [PubMed]
- Yin XT, Zobell S, Jarosz JG, et al. Anti-IL-17 therapy restricts and reverses late-term corneal allorejection. J Immunol 2015;194:4029-38. [Crossref] [PubMed]
- Sharma AK, Mulloy DP, Le LT, et al. NADPH oxidase mediates synergistic effects of IL-17 and TNF-alpha on CXCL1 expression by epithelial cells after lung ischemia-reperfusion. Am J Physiol Lung Cell Mol Physiol 2014;306:L69-79. [Crossref] [PubMed]
- Prakash A, Mesa KR, Wilhelmsen K, et al. Alveolar macrophages and Toll-like receptor 4 mediate ventilated lung ischemia reperfusion injury in mice. Anesthesiology 2012;117:822-35. [Crossref] [PubMed]
- Ding X, Jin S, Tong Y, et al. TLR4 signaling induces TLR3 up-regulation in alveolar macrophages during acute lung injury. Sci Rep 2017;7:34278. [Crossref] [PubMed]
- Xu Z, Sharma M, Gelman A, et al. Significant role for microRNA-21 affecting toll-like receptor pathway in primary graft dysfunction after human lung transplantation. J Heart Lung Transplant 2017;36:331-9. [Crossref] [PubMed]
- Yang Z, Sharma AK, Linden J, et al. CD4+ T Lymphocytes Mediate Acute Pulmonary Ischemia-Reperfusion Injury. J Thorac Cardiovasc Surg 2009;137:695-702. [Crossref] [PubMed]
- Bobadilla JL, Love RB, Jankowska-Gan E, et al. Th-17, Monokines, Collagen Type V, and Primary Graft Dysfunction in Lung Transplantation. Am J Respir Crit Care Med 2008;177:660-8. [Crossref] [PubMed]
- Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 2007;117:3498-506. [Crossref] [PubMed]
- Diamond JM, Porteous MK, Cantu E, et al. Elevated Plasma Angiopoietin-2 Levels and Primary Graft Dysfunction after Lung Transplantation. PLOS ONE 2012;7:e51932. [Crossref] [PubMed]
- Fisher AJ, Donnelly SC, Hirani N, et al. Elevated Levels of Interleukin-8 in Donor Lungs Is Associated with Early Graft Failure after Lung Transplantation. Am J Respir Crit Care Med 2001;163:259-65. [Crossref] [PubMed]
- Hamilton BC, Kukreja J, Ware LB, et al. Protein biomarkers associated with primary graft dysfunction following lung transplantation. Am J Physiol Lung Cell Mol Physiol 2017;312:L531-41. [Crossref] [PubMed]
- Veldhuizen RA, Lee J, Sandler D, et al. Alterations in pulmonary surfactant composition and activity after experimental lung transplantation. Am Rev Respir Dis 1993;148:208-15. [Crossref] [PubMed]
- Amital A, Shitrit D, Raviv Y, et al. The use of surfactant in lung transplantation. Transplantation 2008;86:1554-9. [Crossref] [PubMed]
- Machuca TN, Collaud S, Mercier O, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2015;149:1152-7. [Crossref] [PubMed]
- Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion--pushing the limits. Am J Transplant 2012;12:1839-47. [Crossref] [PubMed]
- Boffini M, Ricci D, Bonato R, et al. Incidence and severity of primary graft dysfunction after lung transplantation using rejected grafts reconditioned with ex vivo lung perfusion. Eur J Cardiothorac Surg 2014;46:789-93. [Crossref] [PubMed]
- Fildes JE, Archer LD, Blaikley J, et al. Clinical Outcome of Patients Transplanted with Marginal Donor Lungs via Ex Vivo Lung Perfusion Compared to Standard Lung Transplantation. Transplantation 2015;99:1078-83. [Crossref] [PubMed]
- Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant 2015;15:993-1002. [Crossref] [PubMed]
- Wallinder A, Ricksten SE, Silverborn M, et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case-control study. Eur J Cardiothorac Surg 2014;45:40-4; discussion 4-5. [Crossref] [PubMed]
- Wigfield CH, Cypel M, Yeung J, et al. Successful emergent lung transplantation after remote ex vivo perfusion optimization and transportation of donor lungs. Am J Transplant 2012;12:2838-44. [Crossref] [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- TransMedics. International Randomized Study of the TransMedics Organ Care System (OCS Lung) for Lung Preservation and Transplantation (INSPIRE). 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT01630434
- TransMedics. International EXPAND Lung Pivotal Trial (EXPANDLung). 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT01963780


