
Transbronchial needle aspiration: where are we now?
Introduction
Lung cancer, due to its aggressive and heterogeneous nature, has been recognized as the leading cause among cancer-related motility and mortality worldwide, with an average 15% five-year survival rate (1). In United States, there are approximate 220,000 newly diagnosed cases every year (2). In the past decades, despite abundant advances in the treatment of lung cancer, including surgical, radiotherapeutic, chemotherapeutic and other novel therapeutic approaches, the prognosis of lung cancer remains poor. Smoking is the predominant risk of this malignant disease, early detection and staging is the principle step for clinical managements and outcomes, which especially benefits the patients who are candidates for surgical resection. To date, TNM system has been employed and well accepted in the staging of lung cancer (3-5). More importantly, the involvement of mediastinal lymph nodes, referring to the stage of N designator in TNM system, usually becomes the determinant factor for treating strategy.
Bronchoscopy is a routine method used for diagnostic and therapeutic procedures performed within the lungs. It allows direct visualization within the lumen of the upper airway and the tracheobronchial tree including subsegmental bronchi. Bronchoscopy is utilized in sampling of the respiration tract secretions and cells, and biopsy of the airway, lung, and mediastinal structures. Since the introduction of transbronchial needle aspiration (TBNA) in flexible bronchoscopy in 1983, conventional TBNA (cTBNA) has been technically well-established and expanded its role in diagnosis and staging of lung cancer. Moreover, recently emerged ultrasound-guided TBNA (EBUS-TBNA) is reported to reveal higher yield in most lymph nodes stations with lower complication rate compared to cTBNA (6-9), though it remains controversial (10). However, it leaves the questions open as to the relationship of the two techniques, whether it is appropriate to advocate endobronchial ultrasound as the standard care in all lymph nodes sampling and what is the value of cTBNA in current stage. In this review, we aim to address these critical issues by comparing the instruments, anatomy, and technique of cTBNA with EBUS-TBNA.
Up to now, cTBNA has been revolutionized to access mediastinal and hilar adenopathy and masses, allowing for moderately invasive approach to achieve samples accurately. The success of cTBNA relies on a thorough understanding of anatomy, including mediastinal structures and visualized intraluminal landmarks which would navigate the operator to the puncture site, and even more importantly, adequate training. On the other hand, there is no doubt that the advent of endobronchial ultrasound is another milestone in the development of TBNA, making the sampling real-time visible, facilitating the localization of targeting lymph nodes, potentiating the successful rate of efficient passes. Nevertheless, both cTBNA and EBUS-TBNA have their own limitations. For example, since cTBNA is a fairly blind technique, it might exhibit low yield in absence of systemic training; also, the needle for cTBNA is relatively hard to control and sometimes requires a three hand procedure; additionally, it is difficult to assess small lymph nodes. For EBUS-TBNA, owing to the size of EBUS scope itself, it appears to be more invasive and need to be performed under general anesthesia in operating room. Concomitantly, the procedures usually ask for two scopes: one regular scope for airway survey, the other EBUS scope for TBNA. Moreover, the price of EBUS set up might not be affordable for most hospitals and that becomes a thorny barrier for the popularization of EBUS-TBNA worldwide.
Anatomy
No matter cTBNA or EBUS-TBNA, thorough understandings of thoracic anatomy are most critical upon TBNA performance. TBNA will not be effective unless the appropriate puncture site is selected. Fortunately, pulmonary lymph nodes anatomy is pretty constant and could be recognized by landmarks in the airway. In order to better understand the location of lymph nodes, Dr. Ko-Pen Wang proposed a map of the mediastinal and hilar lymph node stations for TBNA biopsy with CT and endobronchial correlations, identifying 11 lymph node stations which are consistently involved with metastatic tumor in areas accessible from the airways (11).
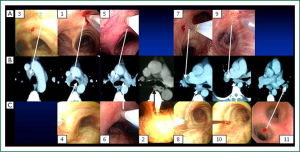
Detailed descriptions of the locations and puncture sites of 11 lymph nodes stations have been well-characterized before (11). Briefly, 11 stations can be categorized into 3 groups: carina region, sub-carina region and hilar region (Figure 1). Although Wang’s lymph node map is closely correlated with the lymph node map proposed by International Association for the Study of Lung Cancer (IASLC), it demonstrates advantages over IASLC’s. More specifically, most IASLC lymph node stations are defined by the anatomic landmarks that could only be identified on imaging studies or surgery, such as aorta, pulmonary vein and esophagus. The clear relationships of all these structures are really complicated to be figured out for the bronchoscopist during TBNA procedures even under EBUS, while Wang’s map identifies different stations by visualized airway branching as the landmarks correlating with chest CT, which could be easily recognized by operators. In addition, instead of a general concept of certain region in IASLC map, Wang’s map points out specific sites for puncture which tremendously facilitates both cTBNA and EBUS-TBNA at a practical standpoint. Whilst, the involvement of lymph nodes estimated based on IASLC map revised from Naruke map and Mountain-Dresler modification of the ATS map, represents the N descriptor in TNM classification of malignant tumors (12). Obviously, it is absolutely imperative for the bronchoscopist to be familiar with IASLC map as well so as to improve the alignment of TNM stage with prognosis and, in certain subsets, with treatments. Due to the pivotal values of Wang’s map in practical use and IASLC map in TNM staging, understanding of the correlations between two maps appears to be extremely important. In Wang’s map, carina region consists of six stations: 1, anterior carina; 2, posterior carina; 3, right paratrachea; 4, left paratracheal or AP window; 5, right main bronchus; and 6, left main bronchus. Out of six stations, 1, 3, 5 stations and 4, 6 stations correspond to 4L and 4R in IASLC map, respectively; station 2 in conjunction with sub-carina region station 8 (sub-carina, right upper lobe bronchus level) and 10 (subsub-carina, right middle lobe bronchus level), accounts for 7 in IASLC map; hilar region in Wang’s map including station 7 (right upper hilar), 9 (right lower hilar) and station 11 (left hilar) coincide with 11R and 11L in IASLC map.
Instruments
The innovation of EBUS bronchoscope is that it enables physicians to visualize lymph nodes and surrounding vessels in real-time, via ultrasound, while simultaneously viewing the endoscopic image. Due to the addition of ultrasound probe, the design of EBUS scope is less flexible compared to the regular bronchoscope, requiring higher skill of scope handling and causing more invasiveness, meanwhile usually need a second scope for the survey purpose, resulting in more cost and less convenience.
Needles specifically for the EBUS bronchoscopes are produced in 22-gauge (22G) and recently introduced 21-gauge (21G) size. A good body of evidence shows that 21G needle does not add diagnostic benefit compared to 22G needle (13,14). Of note, both sizes of EBUS-needle are stiffer and longer than cTBNA needles that made the catheter easier to be controlled. Also, upon doing the puncture, EBUS-needle catheter is fixed to the scope, avoiding (I) over insertion of needle catheter; (II) the need of fixing the scope near the nose in jabbing technique as mentioned in technique section; (III) the retraction of the catheter into the working channel of the scope caused by ineffective fixation of the catheter at the entrance of scope during push technique. Indeed, all above advantages of EBUS needle are the most common mistakes in cTBNA performances. Whilst, EBUS-TBNA needle is relatively more cumbersome than the cTBNA needles and the need of completely removing the inner stylet before applying suction is a nuisance and a risk of contamination. In contrast, although many variations and sizes have been developed for cTBNA needles, MW-319 and SW-221 needles are recommended in most cases. MW-319, modified from MW-418, is a double lumen retractable needle with a 21G inner needle and a 19G outer needle which could be applied in peripheral or central lesions for both cytology and histology. During operation, the needle is retracted into semitranslucent catheter upon insertion through working channel, push the needle out and lock in big bronchus when the distal end of catheter (metal hub) protrudes beyond the bronchoscope, confirming with the inner needle protruding distal to the beveled tip of the needle. After penetrating the bronchial wall, the inner needle mini-trocar could be withdrawn, allowing the beveled tip to act as a cutting edge and core-out a specimen for histology; or maintain the inner needle in and then apply suction directly without retraction for cytology biopsy. A more recent upgrade on top of MW series is attaching the needle to a spring to offer greater flexibility and the development of momentum for enhanced puncture force. SW-221 is one of the needles from this series. SW-221 with a single lumen design has a more flexible inner catheter to support puncture of peripheral and central areas, whereby partially retracting the inner stylet which makes the catheter less stiff, resulting in more possibilities to reach apical or superior segmental lesions to carry out the biopsy. Also, by improving the instruments and technique, a hybrid method of cTBNA is developed via using a fixer as a third hand to eliminate the above mentioned common mistakes for beginner. More importantly, it is noteworthy to mention that both MW-319 and SW-221 needles as well as the fixer could be applied in some of the EBUS-scopes that bring more options for the bronchoscopists under different situations (Video 1). Overall, a wide variety of flexible needle types and variations are available for cTBNA. Continued emphasis and focus on development of simpler, easier to use and more effective needles for TBNA with or without EBUS bronchoscopes is needed.
Methodology
The technique of CTBNA and EBUS-TBNA for cytology and histology specimens is basically similar with minor modification based on the instruments used and location to be sampled. Actually, the elegance and simplicity of TBNA is most evident in the methodology for performing the procedure. The main reasons for low yield could be attributed to poor penetration, inadequate angulation and wrong puncture site despite sufficient penetration and angulation. Therefore, successful penetration is indispensable in TBNA. Below are the major four methods of penetration technique. (I) jobbing method: while the scope is fixed at nose or mouth by assistant, thrust the needle with a quick and firm job to the catheter; (II) pushing method: once the needle is advanced in position, fix the catheter by fixer or index finger, gradually push the scope with stable force until the needle is completely inserted; (III) hub against wall method: maneuver the bronchoscope to the location of interest, advance the catheter with retracted needle out until the metal hub reach the target, then hold firmly as the needle is pushed out to penetrate the bronchial wall. This is the standard insertion method for EBUS needle, while can still be applied in cTBNA needles; (IV) cough method: occasionally, when above methods fail to penetrate the bronchial wall, ask patient to cough which may facilitate the insertion. These four methods are independent but also interrelated. In clinical practice, circumstances alter cases. Good penetration makes successful TBNA.
Mediastinoscopy, percutaneous needle aspiration (PCNA) and esophageal ultrasound-guided fine needle aspiration (TENA)
In addition to cTBNA and EBUS-TBNA, several other techniques are available to evaluate mediastinal, hilar lymph nodes and peripheral lesions, including mediastinoscopy, video-assisted thoracic surgery, TENA, and PCNA. Not all of these procedures are capable of accessing to all lymph node stations, and each is associated with its own risk/benefit profile (15,16).
Traditionally, mediastinoscopy is considered to be the gold standard tool for the management of non-small cell lung cancer with a pooled diagnostic sensitivity of 78-81%, similar to cTBNA but lower than EBUS-TBNA (17). Of note, mediastinoscopy can only be used to investigate the nodal stations 1-4 and 7 in IASLC map which represent carina region in Wang’s map. With identical sensitivity of TBNA, but limited assessment capability and higher invasiveness and risk, mediastinoscopy is no longer the first candidate in lung cancer diagnosis and staging. On the other hand, PCNA is another classical technique used for decades in diagnosing pulmonary lesions. Since it was first performed in lung carcinoma patient in 1886, the major concerns regarding PCNA are its diagnostic sensitivity and complication rate. With the development of visualization technique under CT guidance, the reported sensitivity of PCNA is more than 80% (18), and moreover recent data support the pivotal role of PCNA in the diagnosis and management of small (<1 cm) pulmonary nodules (19,20). Nevertheless, PCNA exhibits significantly higher complication rate, including pneumothorax (~20%), bleeding and air embolism. In general, PCNA is recommended in the situations of negative TBNA, suspicious benign disease, small and peripheral lesions and sub-aortic or para-aortic lymph nodes where both cTBNA and EBUS-TBNA are hard to access.
An increasing body of studies suggested TENA, a recently emerged alternative for primary mediastinal staging of lung cancer, is a safe, promising and noninvasive tool which improves the preoperative staging, especially for the initial estimation (21-23). Moreover, combination of TENA in line with TBNA could provide better diagnostic accuracy than either one alone and totally replace the use of mediastinoscopy as well as avoid unnecessary thoracotomies (22,24-27). Actually, TENA has been well evident to reveal advantage in sampling lymph node at aortopulmonary window (station 4 L) and sub-carina region (stations 7 and 8), whereas pretracheal and hilar lymph nodes are out of reach. However, all of these stations are accessible for TBNA. In our experience, taking patient’s benefit into account, TENA should only be carried out in the situation where the lymph node stations are difficult or are not available by TBNA. If these groups of lymph nodes are the only lymph nodes involved, the patient can go through TENA directly so as to maximize the benefit/cost ratio (28).
Conclusions
TBNA is an ideal approach suited to detect pretracheal and hilar nodes and is critically important for diagnosing, evaluating the extent of the lung cancer and planning optimal treatments. Conceptually, with the induction of online visualization by EBUS, it turns out to be more accessible and accurate to sample the small lymph nodes. However, for the time being, due to the lower affordability, simpler technique but comparable yield, cTBNA will continue to serve as an appealing tool for the diagnosis and staging of lung cancer. Adequate training is essential for both cTBNA and EBUS-TBNA. We speculate these two techniques are not competitive but complementary, judging the indications of patients for different technique would be a raising issue applied for bronchoscopists.
Acknowledgements
K. P. Wang designed the study and gave final approval of the manuscript; Y. Xia drafted the manuscript and revised it critically for important intellectual content.
Disclosure: The authors declare no conflict of interest.
References
- DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin 2013;63:151-66. [PubMed]
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [PubMed]
- Alberts WM. Introduction to the Third Edition: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:38S-40S.
- Rami-Porta R. Reflections on the revisions in the international system for staging lung cancer. Chest 1998;113:1728-9. [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [PubMed]
- Okamoto H, Watanabe K, Nagatomo A, et al. Endobronchial ultrasonography for mediastinal and hilar lymph node metastases of lung cancer. Chest 2002;121:1498-506. [PubMed]
- Herth F, Becker HD, Manegold C, et al. Endobronchial ultrasound (EBUS)--assessment of a new diagnostic tool in bronchoscopy for staging of lung cancer. Onkologie 2001;24:151-4. [PubMed]
- Arslan Z, Ilgazli A, Bakir M, et al. Conventional vs. endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal lymphadenopathies. Tuberk Toraks 2011;59:153-7. [PubMed]
- Tremblay A, Stather DR, Maceachern P, et al. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340-6. [PubMed]
- Shannon JJ, Bude RO, Orens JB, et al. Endobronchial ultrasound-guided needle aspiration of mediastinal adenopathy. Am J Respir Crit Care Med 1996;153:1424-30. [PubMed]
- Wang KP. Staging of bronchogenic carcinoma by bronchoscopy. Chest 1994;106:588-93. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Yarmus LB, Akulian J, Lechtzin N, et al. Comparison of 21-gauge and 22-gauge aspiration needle in endobronchial ultrasound-guided transbronchial needle aspiration: results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013;143:1036-43. [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [PubMed]
- De Leyn P, Lardinois D, Van Schil PE, et al. ESTS guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg 2007;32:1-8. [PubMed]
- Wang KP. Transbronchial needle aspiration and percutaneous needle aspiration for staging and diagnosis of lung cancer. Clin Chest Med 1995;16:535-52. [PubMed]
- Medford AR, Bennett JA, Free CM, et al. Mediastinal staging procedures in lung cancer: EBUS, TBNA and mediastinoscopy. Curr Opin Pulm Med 2009;15:334-42. [PubMed]
- Gasparini S, Ferretti M, Secchi EB, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses. Experience with 1,027 consecutive cases. Chest 1995;108:131-7. [PubMed]
- Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002;225:823-8. [PubMed]
- Ng YL, Patsios D, Roberts H, et al. CT-guided percutaneous fine-needle aspiration biopsy of pulmonary nodules measuring 10 mm or less. Clin Radiol 2008;63:272-7. [PubMed]
- Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: a systematic review and metaanalysis. Chest 2007;131:539-48. [PubMed]
- Annema JT, Versteegh MI, Veseliç M, et al. Endoscopic ultrasound added to mediastinoscopy for preoperative staging of patients with lung cancer. JAMA 2005;294:931-6. [PubMed]
- Tournoy KG, De Ryck F, Vanwalleghem LR, et al. Endoscopic ultrasound reduces surgical mediastinal staging in lung cancer: a randomized trial. Am J Respir Crit Care Med 2008;177:531-5. [PubMed]
- Annema JT, van Meerbeeck JP, Rintoul RC, et al. Mediastinoscopy vs endosonography for mediastinal nodal staging of lung cancer: a randomized trial. JAMA 2010;304:2245-52. [PubMed]
- Herth FJ, Krasnik M, Kahn N, et al. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest 2010;138:790-4. [PubMed]
- Hwangbo B, Lee GK, Lee HS, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest 2010;138:795-802. [PubMed]
- Vilmann P, Krasnik M, Larsen SS, et al. Transesophageal endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy: a combined approach in the evaluation of mediastinal lesions. Endoscopy 2005;37:833-9. [PubMed]
- Wang KP, Feller-Kopman D, Mehta A, et al. Endobronchial ultrasound and esophageal ultrasound: just because we can, does not necessarily mean we should. Chest 2011;140:271-2; author reply 272-3. [PubMed]


