Doxycycline attenuates paraquat-induced pulmonary fibrosis by downregulating the TGF-¦Â signaling pathway
Introduction
Paraquat (PQ) is an herbicide that remains widely used in agriculture. One of the major known toxicities in humans is progressive and irreversible pulmonary fibrosis (PF) (1). Human PF is a chronic and fatal disease; its pathological characteristics include fibroblast proliferation, excessive deposition of collagens and other extracellular matrix (ECM) proteins, and disruption of lung structure and function that lead to respiratory failure (2-4).
Fibroblasts and myofibroblasts play key roles in the PF process (5). These cells are involved in the repair of aberrant tissues and deposition of ECM components. Fibroblasts and myoblasts proliferate during the progression of fibrosis, leading to the disorder of epithelial-mesenchymal cells. And in the process of PF alveolar epithelial cells can be induced express proteins associated with fibroblasts and myofibroblasts, and approximately 36% of fibroblasts in renal fibrosis originate from tubular epithelial cells from the injury site (6). Additionally, 42.60% of N-cad-positive cells are derived from pulmonary epithelial cells in cases of SWCNT (Single walled carbon nanotubes)-induced PF (7). In patients with human idiopathic pulmonary fibrosis (IPF), approximately 80% of alveolar epithelial cells in the fibrotic loci express the mesenchymal marker alpha smooth muscle actin (α-SMA) (8). These phenomena are considered to be features of the epithelial-mesenchymal transition (EMT), lead to the loss alveolar epithelium function and can increase the disorder of epithelial-mesenchymal cells. EMT plays an important role in tissue development during embryogenesis, and similar cell transitions occur in pathological processes such as fibrosis (9,10). These findings suggest that EMT may be a potential key step for PF, and the inhibition of EMT may be a useful way to attenuate PF.
Doxycycline (Doxy) is a tetracycline antibiotic used to treat infectious diseases (11,12). In addition, the following functions have been reported for Doxy: MMP inhibition, anti-inflammatory properties, and anti-invasive, anti-proliferative, anti-angiogenic, and pro-apoptotic functions (13-15). Recent studies demonstrated that Doxy attenuates bleomycin-induced PF by decreasing inflammation and inhibiting both growth factors and MMPs (16,17). In this study, we report the potential of using Doxy to treat PQ-induced PF and describe the role of Doxy in regulating EMT and TGF-β1.
Methods
Chemicals and reagents
Doxy hydrochloride (purity >91%) was provided by Kaifeng Pharmaceutical (Group) Co., Ltd. (Kaifeng, China). Dexamethasone (purity >98%) was purchased from Dalian Meilun Biological Technology Co., Ltd. (Dalian, China). PQ (purity >99%) was purchased from J&K Scientific Ltd. (Beijing, China). Doxy hydrochloride, dexamethasone, and PQ were dissolved in 0.9% saline before administration. 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sangon Biotech (Shanghai, China).
Cell culture
The human lung cancer cell line A549 was purchased from KeyGen Biotech (Nanjing, China). The cells were cultured in medium supplemented with 10% heat-inactivated (56 °C, 30 min) fetal calf serum (HyClone, USA) and maintained at 37 °C with 5% CO2 in a humidified atmosphere.
Animals
Seventy-two adult Kunming mice (weighing 20–25 g) were purchased from the China Institutes for Food and Drug Control (SYXK 2012-0003). The animals were acclimated to the laboratory for at least 7 days before use in the experiments. The animal care and use complied with the Provisions and General Recommendation of the Chinese Experimental Animals Administration Legislation. The study protocol was approved by the Institutional Ethical Committee of Tianjin International Joint Academy of Biomedicine (permit Number: SYXK 2017-0003).
PQ-induced PF in mice
The PF mouse model was established by a single intragastric administration of PQ at 100 mg/kg body weight. The mice were randomly divided into the following groups: control group, model group, dexamethasone (DEX, 1 mg/kg)-treated group, and Doxy (60, 30, and 15 mg/kg)-treated group. DEX and Doxy were given by intragastric administration every two days for 21 days beginning 7 days after PQ administration. The mice in the control group were given an equal volume of vehicle (0.9% saline) on the same schedule by intragastric administration. The animal body weights were measured once daily. The mice were sacrificed on day 28 with excess chloral hydrate hydrochloride anesthesia. The lungs were then removed weighed. The pulmonary coefficient was calculated as lung weight/body weight ×100%.
For early stage inflammatory response analysis, DEX (1 mg/kg) and Doxy (60, 30, and 15 mg/kg) were given every day from the beginning day of PQ administration and the mice were sacrificed at day 7.
Hydroxyproline (HYP) analysis
The left lung was used to measure the content of HYP. The content of lung HYP was assayed using the chloramine-T method (18). The data are shown in as micrograms of HYP per milligram of lung tissue (µg/mg).
Histological examination
After the lungs were removed, the right lung tissue was immediately fixed in 4% paraformaldehyde. The tissues were then dehydrated and embedded in paraffin. We prepared 4-µm-thick paraffin sections and stained the tissues with hematoxylin and eosin (H&E) for visual examination of morphological changes by microscopy. We also performed Masson’s trichrome staining to evaluate fibrosis (collagen fibers). The process was performed following the manufacturers’ standard protocols. The samples were inspected using an Olympus microscope (Tokyo, Japan). The H&E staining results were scored for the following parameters: epithelial proliferation, alveolitis, edema, inflammatory cell infiltration, and interstitial fibrosis. The results from Masson's trichrome staining were scored for the levels of ECM. The criteria for grading were as follows: grade 0, normal; grade 0.5, slight; grade 1, mild; grade 2, moderate; and grade 3, severe.
Immunohistochemical staining assay
The paraffin sections (4 µm thick) of mouse lungs were deparaffinized prior to antigen retrieval in citrate buffer solution heated by a microwave to 90 °C for 15 min. The sections were blocked with normal goat plasma for 20 min at 37 °C and then incubated overnight at 4 °C with primary antibodies against the following: α-SMA antigen (dilution 1:200; Affinity), SPC antigen (dilution 1:100; Affinity), vimentin antigen (dilution 1:50; Affinity), and E-cadherin antigen (dilution 1:100; Zhongshan). The sections were incubated for 20 min with a streptavidin-biotin-peroxidase complex, and then the sections were incubated with secondary antibody for 30 min at 37 °C. Diaminobenzidine and hematoxylin were used for color development and counterstaining, respectively. The resulting slides were examined under a light microscope at a photodocumentation facility and the immunohistochemical staining index was quantitated by blinded observers. The number of positively stained cells was calculated from twenty different 400× magnified fields for each tissue section.
Enzyme-linked immunosorbent assay (ELISA) assay
The mouse blood was collected by retro-orbital bleeding, and the plasma was prepared by centrifugation and then frozen at −80 °C for subsequent analysis. The levels of mouse TGF-β1, TNF-α, IL-4, and IFN-γ were assayed using an ELISA kit (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). The procedure was performed according to the manufacturer’s instructions.
Bronchoalveolar lavage fluid (BALF) collection
The trachea of mice was cannulated and lavaged three times with 1 ml cold PBS. BAL fluid samples were centrifuged at 1,000 rpm for 10 min and the cell-free supernatants were collected for ELISA assay. The cell pellets were resuspended in 200 µl PBS and total cell number was counted with a hemocytometer. Cytospin preparations of BAL fluid cells were stained with H&E and viewed under light microscopy for inflammatory cell differential.
Multidimensional liquid chromatography tandem mass spectrometry
A549 cells (4×103 cells/mL) were seeded in a 100-mm dish. When the plate was 70–80% confluent, we treated the cells with 5 ng/mL recombinant human TGF-β1 for 3 hours. The cells were then cultured in the presence or absence of Doxy hydrochloride (3.4 µM) for 24 h. The cells were then lysed, and the samples were examined using multidimensional liquid chromatography-tandem mass spectrometry.
Statistical analysis
All data are expressed as the mean ± standard deviation (SD). The statistical comparisons among treatment groups were performed using one-way ANOVA. All analyses were performed using SPSS 17.0 statistical software. Differences between experimental groups of P<0.05 were considered significant.
Results
Doxy attenuated PQ-induced PF in mice
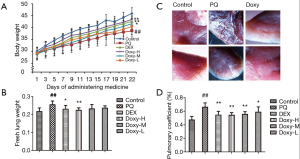
As shown in Figure 1A, the average body weight was lower in the PQ-treated group than in the control and Doxy-treated groups. The average body weight tended to recover following treatment with Doxy (n=12, P<0.05 for each model group). The average body weight was higher in the Doxy-treated group than in the DEX-treated group (Figure 1A). The normal lung tissue from the control group mice was pink. However, the lungs of mice in the PQ model group were rigid, smaller in size, and displayed a relatively dark color. The Doxy treatment reversed the change in the lung appearance in the Doxy-treated groups. The treatment with 60 mg/kg Doxy restored the lung color (Figure 1B). The lungs of the model group mice showed severe edema, higher lung weight, and higher pulmonary coefficients. As shown in Figure 1C,D, the Doxy treatments significantly reduced both the lung weight and pulmonary coefficients in a dose-dependent manner. The lung weight and pulmonary coefficient were lower in the high-dose group (60 mg/kg) than in the DEX-treated group. These results indicate that Doxy treatment can reduce the extent of lung oedema.
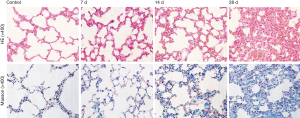
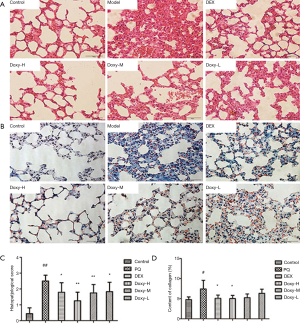
Next we confirmed the pathologic changes after PQ modeling. Compared with the control group, little quantity of collagen deposition in lung tissue at day 7 and collapsible alveolar spaces, fibroblasts proliferation and more collagen deposition appears form day 14 to day 28 in the experimental group (Figure S1). Figure 2 shows the pathologic changes in the lung. The sections of the control group show intact and clear lung structures, and no pathologic changes were observed (Figure 2A). A limited quantity of collagen was found (Figure 2B). However, in the model group, the alveolar walls were thickened, the alveolar spaces were collapsed, and the alveolar structures were difficult to identify (Figure 2A,B). This group also exhibited excess fibroblast proliferation and more collagen than the control group. These results clearly indicated that severe PF can be induced by PQ. The DEX and Doxy treatments improved the alveolar structure of the lung compared with that in the model group, and the lung structure and collagen content were similar to those of the control group. The histopathological score and collagen content suggest that the Doxy-H treatment had the best efficacy, with superior results compared with those of the DEX-treated group. The efficacy of Doxy was also dose dependent.
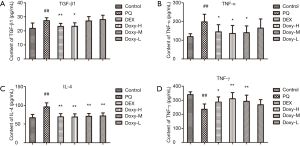
Doxy can inhibit the expression of inflammatory cytokines in PF
The inflammatory cytokines TNF-α, IL-4, TGF-β1, and IFN-γ in the plasma were evaluated using ELISA to determine whether Doxy affects the inflammatory response in PQ-induced PF. As shown in Figure 3, within three weeks of PQ treatment, the levels of inflammatory cytokines, such as TNF-α, IL-4, and TGF-β1, in the plasma were significantly higher in the model mice than in the control group. Conversely, the plasma level of IFN-γ was lower. However, the treatments with DEX and Doxy reversed the changes in these cytokines and reduced the levels of TNF-α, IL-4, and TGF-β1 while increasing the levels of IFN-γ. In addition, the effect of Doxy treatment was also dose dependent.
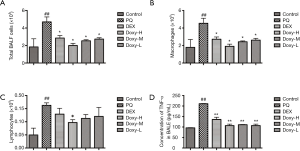
Doxy attenuated inflammatory response at early stage of PQ-induced lung injury
We investigated the role of Doxy in the inflammatory response to PQ-induced lung injury at early stage. We observed an increase in total inflammatory cells in the BALF after PQ treatment. However, a significant reduction in total inflammatory cells was observed in DEX and Doxy-treated group as compared with controls (Figure 4A). Similar effect of DEX and Doxy were observed in macrophages and lymphocytes counting results (Figure 4B,C) and the effect of Doxy treatment was dose dependent. The treatment of DEX and Doxy reduced the expression level of TNF-α in BALF (Figure 4D). These data suggest Doxy could reduce inflammatory response at early stage of PQ-induced lung injury.
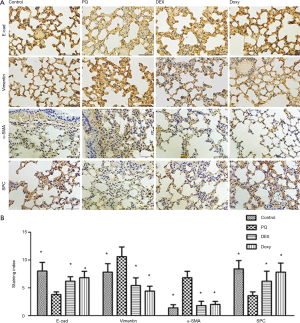
Doxy potentially regulates the PQ-induced disorder of epithelial-mesenchymal cells
We examined several markers of epithelial and mesenchymal cells to determine whether Doxy affects the transition of alveolar epithelial cells to fibroblasts and the balance of epithelial-mesenchymal cells. As shown in Figure 5, PQ treatment reduces the expression levels of E-cad and SPC, which are markers of epithelial cells. However, PQ increased the levels of the mesenchymal cell markers vimentin and α-SMA, which suggests that the cells are similar to fibroblast stem cells. When the PQ-pretreated alveolar epithelial cells were treated with Doxy and DEX, the expression levels of E-cad and SPC were restored, and the levels of vimentin and α-SMA were reduced. The treatments with 60 mg/kg Doxy (Doxy-H group) showed that the Doxy treatment was more effective than the DEX treatment in regulating EMT process.
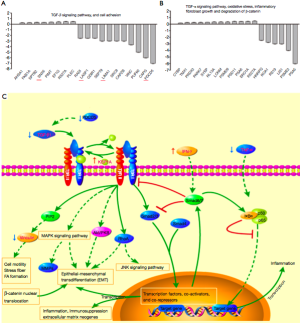
Doxy attenuates PQ-induced PF by regulating numerous related proteins
We conducted proteomic analyses to determine the expression levels and activities of key proteins regulated by Doxy in TGF-β1-treated A549 cells. The results (Figure 6A,B) showed that Doxy regulates amount of proteins to effect on TGF-β signal transduction, cell-cell adhesion, TNF-α pathway, Oxidative stress, inflammatory response, fibroblast growth and degradation of β-catenin. And we draw the map of TGF-β signaling pathway according to database of UniProt (Figure 6C).
Discussion
This study demonstrated that Doxy can suppress PQ-induced inflammation, excessive expression of collagen, and related fibroblast proliferation and migration to alleviate pathologic changes. Doxy can reverse the downregulation of E-cadherin and SPC and the upregulation of vimentin and α-SMA induced by PQ in vivo. These changes can reduce the EMT and restore the balance of epithelial-mesenchymal cells. In addition, the Smad and non-Smad TGF-β pathways can also be inhibited by Doxy, as shown by the changes in the protein expression of FKB1A and SNX6.
The alveolar epithelium is an important component of lung tissue, is composed of type I and type II epithelial cells (AECI and AECII, respectively) and performs the functions of gas exchange and surfactant secretion (19). Previous studies reported that the alveolar epithelium is a key factor in the development of interstitial lung disease (ILD) and that AECIIs play critical roles in repairing the disrupted surface (9,20,21). In this study, we observed that PQ treatment in pulmonary epithelial cells can decrease the expression of epithelial markers and increase the expression of mesenchymal markers. These changes are often associated with EMT. The expression levels of epithelial markers decreased, and the expression levels of mesenchymal markers increased. These changes may be caused by EMT but could also indicate the disorder of epithelial-mesenchymal cells. Furthermore, these changes may lead to a loss of function in the alveolar epithelium and to accelerated ECM deposition. In this study, we observed the upregulation of E-cadherin and SPC and the downregulation vimentin and α-SMA, indicated Doxy inhibited EMT during the PQ-induced PF and restored the balance of epithelial-mesenchymal cells. TGF-β1 is a key cytokine in the process of fibrogenesis, plays a pivotal role in the fibrosis of many different organs (22), and is a potent chemoattractant for fibroblasts. TGF-β1 can induce alveolar epithelial cells to undergo EMT by causing morphological changes in cells and the upregulation of mesenchymal markers and downregulation of epithelial markers (23-25). Thus, TGF-β1 is considered a “master switch” of the fibrosis process (26). Previous studies showed that Doxy did not reduce TGF-β-induced Smad phosphorylation and acted via a non-Smad pathway (16). In this study, we show that Doxy inhibits both the Smad-mediated and non-Smad mediated pathways of the TGF-β signaling pathway.
The immunophilin peptidyl-prolyl cis-trans isomerase (FKB1A) is a protein that binds to type I receptor (27). Previous studies have shown that FKB1A overexpression can inhibit ligand-induced type I receptor phosphorylation and inhibits the TGF-β signaling pathway (28). Sorting nexin 6 (SNX6) interacts with the TGF-β family of receptor serine-threonine kinases, and SNX6 overexpression interferes with TGF-β signaling (29). In this study, we showed that Doxy upregulates FKB1A and SNX6. Doxy was found to regulate FKB1A and SNX6 in addition to other proteins to inhibit the Smad and non-Smad TGF-β pathway, oxidative stress, and inflammation. This finding further suggests that Doxy restores the balance of epithelial-mesenchymal cells by inhibiting the TGF-β signaling pathway and suppressing oxidative stress and inflammation to alleviate epithelial cell injury.
Inflammation plays a role in disease genesis and progression in both human IPF and murine models of PF (30). In this study, we analyzed the effect of Doxy on inflammation by evaluating the levels of inflammatory cytokines in mouse plasma. We found that Doxy reduced the expression levels of TNF-α, IL-4 and TGF-β1 but increased the levels of IFN-γ. In addition, TNF-α is a proinflammatory cytokine associated with PF (31,32). TNF-α can also induce TGF-β1 expression in lung fibroblasts at the transcriptional level via AP-1 activation and can enhance EMT in combination with TGF-β through IKKβ, which is directly controlled by TAK1 and JNK-2 (33,34). Doxy can suppress the TNF-α pathway to decrease inflammation and improve the antioxidant capacity of cells, ultimately alleviating PF. These results indicate that Doxy can potentially attenuate PF and are consistent with previously reported data (16,17).
In summary, we demonstrated that Doxy inhibits EMT in alveolar epithelial cells and attenuates PQ-induced PF by downregulating the TGF-β signaling pathway and the TNF-α signaling pathway.
Acknowledgements
Funding: This study was supported by Tianjin science and technology innovation system and the condition of platform construction plan (Grant 14TXSYJC00572).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Ethical Committee of Tianjin International Joint Academy of Biomedicine (No. SYXK 2017-0003).
References
- Sun B, Chen Y. Advances in the mechanism of paraquat-induced pulmonary injury. Eur Rev Med Pharmacol Sci 2016;20:1597-602. [PubMed]
- Wynn TA, Ramalingam T. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028-40. [Crossref] [PubMed]
- Ley B, Collard H, Jr K. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431-40. [Crossref] [PubMed]
- Ji Y, Wang T, Wei Z, et al. Paeoniflorin, the main active constituent of Paeonia lactiflora, roots, attenuates bleomycin-induced pulmonary fibrosis in mice by suppressing the synthesis of type I collagen. J Ethnopharmacol 2013;149:825-32. [Crossref] [PubMed]
- White ES, Lazar M, Thannickal V. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol 2003;201:343-54. [Crossref] [PubMed]
- Iwano M, Plieth D, Danoff T, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 2002;110:341-50. [Crossref] [PubMed]
- Chang CC, Tsai M, Huang H, et al. Epithelial-mesenchymal transition contributes to SWCNT-induced pulmonary fibrosis. Nanotoxicology 2012;6:600-10. [Crossref] [PubMed]
- Willis BC, Liebler J, Luby-Phelps K, et al. Induction of Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells by Transforming Growth Factor-β1: Potential Role in Idiopathic Pulmonary Fibrosis. Am J Pathol 2005;166:1321-32. [Crossref] [PubMed]
- Corvol H, Flamein F, Epaud R, et al. Lung alveolar epithelium and interstitial lung disease. Int J Biochem Cell Biol 2009;41:1643-51. [Crossref] [PubMed]
- Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006;172:973-81. [Crossref] [PubMed]
- Akpek EK, Merchant A, Pinar V, et al. Ocular Rosacea: Patient Characteristics and Follow-up. Ophthalmology 1997;104:1863-7. [Crossref] [PubMed]
- Sagar J. Doxycycline in Clinical Medicine. Clin Med Insights Ther 2010;2:133-6. [Crossref]
- Mouratidis PX, Colston K, Dalgleish A. Doxycycline induces caspase-dependent apoptosis in human pancreatic cancer cells. Int J Cancer 2007;120:743-52. [Crossref] [PubMed]
- Saglam F, Celik A, Tayfur D, et al. Decrease in cell proliferation by a matrix metalloproteinase inhibitor, doxycycline, in a model of immune-complex nephritis. Nephrology 2010;15:560-7. [Crossref] [PubMed]
- Qin Y, Zhang Q, Shan L, et al. Doxycycline reverses epithelial-to-mesenchymal transition and suppresses the proliferation and metastasis of lung cancer cells. Oncotarget 2015;6:40667-79. [Crossref] [PubMed]
- Fujita H, Sakamoto N, Ishimatsu Y, et al. Effects of doxycycline on production of growth factors and matrix metalloproteinases in pulmonary fibrosis. Respiration 2011;81:420-30. [Crossref] [PubMed]
- Fujita M, Ye Q, Harada E, et al. Doxycycline attenuated pulmonary fibrosis induced by bleomycin in mice. Antimicrob Agents Chemother 2006;50:739-43. [Crossref] [PubMed]
- Zhang Z, Jian X, Zhang W, et al. Using bosentan to treat paraquat poisoning-induced acute lung injury in rats. Plos One 2013;8:e75943. [Crossref] [PubMed]
- Furuyama A, Kimata K, Mochitate K. Assembly of basement membrane in vitro by cooperation between alveolar epithelial cells and pulmonary fibroblasts. Cell Struct Funct 1997;22:603-14. [Crossref] [PubMed]
- Ley K, Zarbock A. From lung injury to fibrosis. Nat Med 2008;14:20-1. [Crossref] [PubMed]
- Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol-Lung Cell Mol Physiol 2007;293:L525-34. [Crossref] [PubMed]
- Bonniaud P, Kolb M, Galt T, et al. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol 2004;173:2099-108. [Crossref] [PubMed]
- Antony VB, Sahn S, Mossman B, et al. Pleural Cell Biology in Health and Disease. Am Rev Respir Dis 1992;145:1236-9. [Crossref] [PubMed]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004;15:1-12. [Crossref] [PubMed]
- Maniscalco WM, Sinkin R, Watkins R, et al. Transforming growth factor-beta 1 modulates type II cell fibronectin and surfactant protein C expression. Am J Physiol 1994;267:L569-77. [PubMed]
- Sime PJ, O'Reilly K. Fibrosis of the Lung and Other Tissues: New Concepts in Pathogenesis and Treatment. Clin Immunol 2001;99:308-19. [Crossref] [PubMed]
- Wang T, Li B, Danielson P, et al. The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell 1996;86:435-44. [Crossref] [PubMed]
- Chen YG, Liu F, Massagué J. Mechanism of TGFβ receptor inhibition by FKBP12. Embo J 1997;16:3866-76. [Crossref] [PubMed]
- Parks WT, Frank D, Huff C, et al. Sorting Nexin 6, a Novel SNX, Interacts with the Transforming Growth Factor-β Family of Receptor Serine-Threonine Kinases. J Biol Chem 2001;276:19332-9. [Crossref] [PubMed]
- Bringardner BD, Baran C, Eubank T, et al. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal 2008;10:287-301. [Crossref] [PubMed]
- Sullivan DE, Ferris M, Nguyen H, et al. TNF-alpha induces TGF-beta1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J Cell Mol Med 2009;13:1866-76. [Crossref] [PubMed]
- López-Novoa JM, Nieto M. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. Embo Mol Med 2009;1:303-14. [Crossref] [PubMed]
- Yamauchi Y, Kohyama T, Takizawa H, et al. Tumor necrosis factor-alpha enhances both epithelial-mesenchymal transition and cell contraction induced in A549 human alveolar epithelial cells by transforming growth factor-beta1. Exp Lung Res 2010;36:12-24. [Crossref] [PubMed]
- Noguchi S, Yamauchi Y, Takizawa H. Novel therapeutic strategies for fibrotic lung disease: a review with a focus on epithelial-mesenchymal transition. Recent Pat Inflamm Allergy Drug Discov 2014;8:9-18. [Crossref] [PubMed]


