Perforation of myocardial wall and great vessels after cardiovascular interventions—a 5-year analysis
Introduction
During recent years, less invasive procedures have replaced open surgical treatment in many cardiovascular disorders, and the number of interventional therapies is still increasing. Apart from cardiac catheterization, which bears a well-defined low risk of myocardial and vascular wall perforation, pacemaker (PM) and cardioverter-defibrillator lead implantation are the procedures performed most (1). During the past decade, the more demanding transcutaneous aortic valve replacements (TAVR) and percutaneous mitral interventions (MitraClip) have been added to the therapeutic armamentarium in many institutions (2). Even if quality control measures and a rigorous surveillance have been implemented by the authorities especially for TAVR and MitraClip procedures to keep costs and complications low, many other less invasive procedures are much less supervised, and thus only little information exists on their associated complications.
Trauma related penetrating cardiac lesions are rare and carry a high mortality rate, up to 80% has been reported (3,4). In contrast, myocardial wall and vessel perforation associated with diagnostic or therapeutic procedures are much more common but far less dangerous. Yet, pericardial tamponade may rapidly evolve as a life-threatening complication, which requires immediate diagnosis and treatment.
This analysis reports the institutional 5-year experience of patients with suspected iatrogenic myocardial wall or central vessel perforation admitted to a cardiothoracic surgery department in a tertiary care university medical center.
Methods
The institutional ethics committee has approved the retrospective study (reference number 16-104-0041); informed consent has been waived. From April 2011 to March 2016, all patients admitted to the Department of Cardiothoracic Surgery with cardiac or central vessel perforation after interventional procedures were included. Patients with transapical (but not transfemoral) aortic valve implantation (TAVR) were considered surgical cases and were excluded from the study. Likewise, cases with coronary artery perforation during percutaneous coronary interventions (PCI) were omitted as these patients mostly are no surgical candidates. This study analyzed symptoms, treatment mortality, intraoperative findings, and coagulation state at the time of cardiac laceration.
Statistical analysis was performed with GraphPad Prism 6.07 (GraphPad, Inc., San Diego, CA, USA). Descriptive results are shown as mean values with standard deviation. Statistical differences were calculated with non-parametric Mann-Whitney test. P value <0.05 were considered statistically significant.
Results
Demographic data and diagnostics
Forty-four patients (17 male, 27 female) with a mean age of 76±13 years were admitted to our department due to perforation of cardiac structures after an iatrogenic diagnostic or therapeutic intervention. Twenty-seven (61.4%) patients had undergone in-house procedures, whereas 17 (38.6%) patients were transferred from distant hospitals on an urgent or emergency basis.
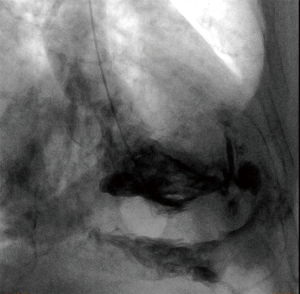
Perforations after in-house procedures (n=27) occurred in nine cases after TAVR, in six cases after placement of permanent pacemakers, in three cases after placement of an extracorporeal life support (ECLS) circuit. In further six cases transcutaneous PM leads (n=3), and pericardiocentesis (n=3) caused perforation. During the investigate period incidence rate of ventricle perforation after TAVR was 1.4% (9 of 642 procedures) and 0.4% (6 of 1,579 procedures) after permanent PM lead placement, respectively. Incidence rates of related procedures from distant hospital were not available. In 22 (50.0%) cases, the diagnosis of a cardiac or vascular lesion was based primarily on echocardiography. Computed tomography imaging established the diagnosis in 7 (15.9%) patients. In one case, perforation of the left ventricle was demonstrated by contrast agent enhancement in the pericardial space after ventriculography during elective diagnostic coronary angiography (Figure 1). In the remaining 14 (31.8%) cases, the lesion responsible for the pericardial effusion or tamponade was verified by direct view during emergency surgery.
Localization
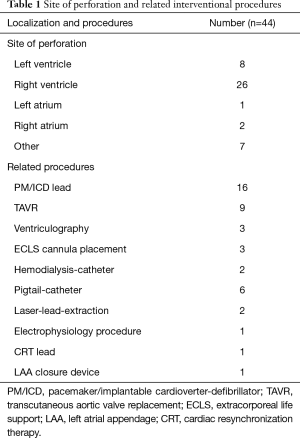
Most myocardial wall perforations were found at the right ventricle (n=26, 59.1%). The left ventricle was involved in 8 (18.2%) patients. Atrial lacerations were seen in three patients only, two on the right side, and one on the left side. A perforation of central venous structures including the brachiocephalic vein (n=2), superior vena cava superior (n=1), and coronary sinus (n=1) was noted in four cases. Three patients developed pericardial effusion after a transfemoral TAVR procedure without evidence of left ventricle perforation. In these cases, a laceration of the aortic valve annulus was assumed. An overview of localization and underlying procedures is shown in Table 1.
Full table
Symptoms and management
Immediate evidence of perforation was apparent in 27 patients. From these, 24 patients underwent immediate therapy and three patients underwent therapy within 24 hours. In five patients with absent acute symptoms, intrapericardial perforation was detected after more than 24 hours, in three patients even after more than one week. Pericardial effusion was evident in 93.2% (n=41) of cases, mean extent on echocardiography was 19±9 mm. However, symptoms of cardiac tamponade including hemodynamic compromise and catecholamine demand were present in 63.6% (n=28) of patients only. Cardiac tamponade dominated in case of left-ventricular laceration, where it was encountered in 7 of 8 (87.5%) patients. In contrast, patients with right ventricular lesions were less likely developing cardiac tamponade (55.6%) as were patients with perforation of central vessels (54.5%).
Extrinsic coagulation as well as platelet counts were comparable in all subgroups [P= not significant (n.s.)]. Only in patients with heparin administration prior to the iatrogenic laceration of the left ventricle, partial thromboplastin time (PTT) was increased (left vs. right ventricle, P=0.0013; left ventricle vs. other laceration, P=0.031). There was no difference for international normalized ratio (INR) and PTT levels (P=n.s.) in patients with and without surgical treatment, even if platelet counts were lower in patients who required an emergency surgical intervention (P=0.044).
Sixteen (36.4%) patients required resuscitation to maintain sufficient circulation. In three cases, hemodynamic stabilization could be achieved only with an ECLS system (veno-arterial ECLS). Twenty-seven patients underwent median sternotomy (61.4%) for open surgical repair (five patients proceeded to sternotomy after initial pericardiocentesis due to persisting hemorrhage and progressive hemodynamic collapse). In all these cases direct closure of the perforated structure was successful. Nine (20.5%) patients underwent percutaneous pericardiocentesis guided by echocardiography. In two cases, a chest tube was placed into the pericardial space by a surgical subxiphoidal access. Due to stable hemodynamic state a pericardial intervention was renounced in 7 (15.9%) cases.
Left ventricular lesions
Eight patients presented with perforation of the left ventricle. Six patients had undergone a transfemoral TAVR procedure before. Two left ventricular lesions were caused by a ventriculography catheter and a guide wire displacement during veno-arterial ECLS placement, respectively. All patients with left ventricular perforation underwent emergency median sternotomy. Despite immediate surgical treatment hospital overall mortality was 75.0% (6 of 8 patients). Five of these patients died following TAVR corresponding to a mortality rate of 83.3% in this patient subset. Pericardial effusion after transfemoral TAVR with suspected lesion of the aortic valve annulus was relieved percutaneously.
Right ventricular lesions
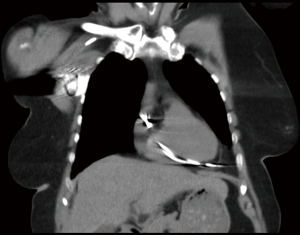
Twenty-six patients suffered from of right ventricular perforation which was mostly attributed to placement of permanent PM or cardioverter-defibrillator leads (n=13, 50.0%) (Figure 2) or to pericardiocentesis with a pigtail catheter (n=6, 23.1%). In three cases, a percutaneous PM lead caused myocardial wall perforation (11.5%). Other right ventricular perforations occurred during diagnostic right heart catheterization (n=2), placement of a double-lumen cannula for veno-venous extracorporeal membrane oxygenation (ECMO) support (n=1), and during an electrophysiological ablation procedure (n=1). The rate of open surgical treatment via sternotomy was 50.0% (n=13). In two of these cases the initial pericardiocentesis required a secondary sternotomy due to persisting tamponade symptoms and ongoing bleeding. In six cases, a percutaneous pericardiocentesis was sufficient to drain the pericardial effusion. Due to stable hemodynamics, seven patients did not require relief of pericardial effusion. All of the latter patients had undergone permanent PM lead placement. Five patients required a new positioning of the right ventricular lead. All patients with laceration of central venous structures underwent open surgical repair.
Mortality and hospital stay
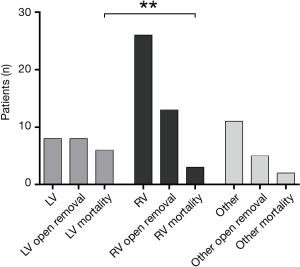
Overall mortality was 25.0% (n=11). Myocardial wall perforation of the left ventricle mortality was associated with a significantly higher mortality than those on the right side with 75.0% vs. 11.5% (P=0.0012) (Figure 3). Mortality of other than ventricular lesions was 20.0%. Most common cause of mortality was circulatory failure (n=5, 11.4%). Three patients died because of secondary multi-organ failure. Two patients suffered from severe hypoxic brain damage caused by a hemodynamic decline. Finally, one patient died because of acute right heart failure related to fulminant pulmonary embolism.
Overall duration of hospital stay in surviving patients was 17±12 days. Length of stay was comparable in patients after open surgery, interventional therapy and without need for therapy (20±13 vs. 13±11 vs. 11±8 days, P=n.s.).
Discussion
The incidence of roughly ten patients per year shows that iatrogenic cardiac perforations are not uncommon complications in a tertiary care hospital. An overall mortality of 25.0% despite immediate and appropriate treatment by well-trained teams underlines the life-threatening character of this complication. Though the average mortality rate is high, not all cardiac perforations are jeopardizing the patient’s life. A good example for the latter is coronary perforation during PCI. With an incidence of 0.12%, cardiac tamponade is a rare event, and only one third of patients require surgical treatment. The vast majority of patients undergoes percutaneous pericardiocentesis or other procedures such as stent placement with a fortunate outcome. Only in case of persistent cardiac tamponade mortality is high (42%) (5).
PM leads penetrating the myocardial wall are more cumbersome, but also here many instances are not recognized because they remain asymptomatic. Thus, the true incidence is probably higher than reported. Sterliński et al. published a perforation rate of 0.5% with no significant difference between the number of perforations between the PM and implantable cardioverter-defibrillator (ICD) implantations (6). A retrospective analysis of 3,815 patients with placement of PM or cardioverter defibrillator figured out a comparable perforation rate of 0.4%. Interestingly, neither type of lead (PM/ICD) nor fixation mode (active/passive) had an effect on perforation (7).
In two of our cases, hemodialysis catheters caused perforation of the brachiocephalic vein. As this vessel is extrapericardially located, assessment of the pericardium integrity can be difficult. CT imaging cannot properly predict the risk of hemorrhage and pericardial tamponade following catheter removal. In cases with stable circulation, removal of the catheter and hemodynamic monitoring is a reasonable option. In face of a large hematoma following perforation, evidence of persisting hemorrhage, or compromised coagulation, open surgical removal is the safer alternative (8).
For the most critical perforating lacerations of the heart, there is hardly any literature available. Life-threatening left ventricular perforations typically occur during transfemoral TAVR procedures, they have been described in up to 7% (9). In a recent meta-analysis from Genereux, cardiac tamponade after TAVR was noted in 0.6–4.6% of patients (pooled estimate 2.7%), mortality rates were not mentioned (10). Main cause of ventricular perforation is the use of a stiff wire, which is essential for proper valve deployment. Likewise, we noticed the highest mortality in this cohort of patients. Despite immediate treatment, five patients with cardiac tamponade and severe hemodynamic depression suffered from multi-organ-failure and cerebral hypoxia. The poor state of the elderly TAVR patients and their comorbidity certainly contribute to the unfortunate results. If resuscitation is necessary during the procedure, survival rates are even worse (11). To keep the procedural risk as low as possible, it is well understandable that the international guidelines recommend to perform TAVR only in units with cardiac surgery units (12). Life-threatening right-sided myocardial wall and vascular perforations happen during placement of veno-venous or veno-arterial extracorporeal support, where a hype is currently going on in Germany (13). Reports of other than vascular lesions after ECLS placement are rare. Perforations after cannula insertion of veno-venous circuits has been described before in a cohort of 94 patients in one case (superior caval vein) and in a cohort of 25 pediatric patients in two cases (right atrium) (14,15). Myocardial lacerations after establishment of veno-arterial support have not been reported. Even with considerable experience in extracorporeal support, the risk for perforation and displacement cannot be excluded entirely. To minimize the risk of a guide wire displacement, transesophageal echocardiography should always be used to control guide wire and cannula positions.
Typically, the occurrence of a cardiac perforation is related to the iatrogenic procedure, and the risk of tamponade is pending. Therefore, immediate assessment via direct imaging, i.e., echocardiography, is crucial. In case of tamponade signs (collapse of the right atrium/ventricle, suppressed collapse of inspiratory inferior vena cava), rapid relief of the pericardial effusion is vital (16). Additional diagnostic imaging is necessary in cases of subacute tamponade with hemodynamic stability and displaced or perforated cardiac devices [PM leads, left atrial appendage (LAA) occlusion devices]. Until definite repair, patient stabilization is required with vasopressor and inotropic support as well as volume resuscitation to maintain sufficient end-diastolic right atrial and ventricular pressures (17). The therapeutic strategy depends on the suspected lesion and patients’ hemodynamic condition: percutaneous intervention should be the first-line procedure, but may be not appropriate in case of left ventricular perforation or rapidly increasing pericardial effusion. Then, early surgical intervention via sternotomy and open repair is the effective therapy. Most left ventricular and most right ventricular perforations require a median sternotomy and open surgical treatment. Left ventricular lesions can rapidly lead to severe hemodynamic compromise and mandate emergency open surgery as only viable rescue option. In contrast, right ventricular lesions may be stabilized without open surgery which renders the rate of sternotomy lower. In general, hemodynamic instability and evidence of an emerging tamponade are the decisive criteria. Most small myocardial wall perforations can be well closed with the heart beating; large lacerations usually mandate the use of extracorporeal circulation and cardioplegic arrest. Interventional procedures to close right ventricular perforations have been devised, but to follow this management the patient needs to be hemodynamically stable (18).
A well-functioning coagulation system is very helpful in sealing small cardiac perforations, i.e., anticoagulation and platelet inhibition is harmful in this situation. Accordingly, it has been shown that platelet inhibition is a risk factor for cardiac tamponade following PM placement (6). In many of our surgically treated patients, we also found low platelet counts, especially in the right ventricular and vascular lesions. Therefore, achieving a physiological coagulation state as first step of treatment is reasonable to stop the hemorrhage as long as the patient is stable. Then, close hemodynamic monitoring and repeated echocardiographic controls are necessary to switch to surgical treatment in time if required.
This analysis has a main limitation. Only patients admitted to the cardiothoracic surgery department with progressively deteriorating circulation were included. Therefore, a substantial number of patients which was managed without admission to the cardiosurgical unit was not included.
Conclusions
Iatrogenic perforation of myocardial wall or central vessel is a rare but life-threatening complication. Despite immediate treatment efforts, mortality is high, particularly after left ventricle laceration. Lack of cardiac tamponade and maintenance of hemodynamic stability are crucial to avoid adverse outcome. Right-sided lacerations have a better outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The University of Regensburg ethical committee has approved the retrospective study (reference number, 16-104-0041).
References
- Kuck KH, Hindricks G, Padeletti L, et al. EHRA white book 2014;2014:197-206.
- Beckmann A, Funkat AK, Lewandowski J, et al. Cardiac Surgery in Germany during 2014: A Report on Behalf of the German Society for Thoracic and Cardiovascular Surgery. Thorac Cardiovasc Surg 2015;63:258-69. [Crossref] [PubMed]
- Kutsukata N, Sakamoto Y, Mashiko K, et al. Morphological evaluation of areas of damage in blunt cardiac injury and investigation of traffic accident research. Gen Thorac Cardiovasc Surg 2012;60:31-5. [Crossref] [PubMed]
- Sassone B, Gabrieli L, Boggian G, et al. Management of traumatic implantable cardioverter defibrillator lead perforation of the right ventricle after car accident: a case report. Europace 2009;11:961-2. [Crossref] [PubMed]
- Fejka M, Dixon SR, Safian RD, et al. Diagnosis, management, and clinical outcome of cardiac tamponade complicating percutaneous coronary intervention. Am J Cardiol 2002;90:1183-6. [Crossref] [PubMed]
- Sterliński M, Przybylski A, Maciag A, et al. Subacute cardiac perforations associated with active fixation leads. Europace 2009;11:206-12. [Crossref] [PubMed]
- Migliore F, Zorzi A, Bertaglia E, et al. Incidence, management, and prevention of right ventricular perforation by pacemaker and implantable cardioverter defibrillator leads. Pacing Clin Electrophysiol 2014;37:1602-9. [Crossref] [PubMed]
- Ko SF, Ng SH, Fang FM, et al. Left brachiocephalic vein perforation: computed tomographic features and treatment considerations. Am J Emerg Med 2007;25:1051-6. [Crossref] [PubMed]
- Masson JB, Kovac J, Schuler G, et al. Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv 2009;2:811-20. [Crossref] [PubMed]
- Généreux P, Head SJ, Van Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol 2012;59:2317-26. [Crossref] [PubMed]
- van Gijn MS, Frijns D, van de Glind EM, et al. The chance of survival and the functional outcome after in-hospital cardiopulmonary resuscitation in older people: a systematic review. Age Ageing 2014;43:456-63. [Crossref] [PubMed]
- Taylor J. ESC/EACTS Guidelines on the management of valvular heart disease. Eur Heart J 2012;33:2371-2. [PubMed]
- Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 2016;42:889-96. [Crossref] [PubMed]
- Pranikoff T, Hirschl RB, Remenapp R, et al. Venovenous extracorporeal life support via percutaneous cannulation in 94 patients. Chest 1999;115:818-22. [Crossref] [PubMed]
- Subramanian S, Vafaeezadeh M, Parrish AR, et al. Comparison of wire-reinforced and non-wire-reinforced dual-lumen catheters for venovenous ECMO in neonates and infants. ASAIO J 2013;59:81-5. [Crossref] [PubMed]
- Bodson L, Bouferrache K, Vieillard-Baron A. Cardiac tamponade. Curr Opin Crit Care 2011;17:416-24. [Crossref] [PubMed]
- Lehmann S, Schröter T, Lehmann A, et al. Pericardial effusion. Differential diagnostics, surveillance and treatment. Chirurg 2011;82:1001-7. Erratum in: Chirurg 2012;83:37. Thomas, S [corrected to Schröter, T]; Leotyev, S [corrected to Leontyev, S]. [Crossref] [PubMed]
- Petrov I, Dimitrov C. Closing of a right ventricle perforation with a vascular closure device. Catheter Cardiovasc Interv 2009;74:247-50. [PubMed]


