Pneumatosis intestinalis after lung transplantation for pulmonary graft-versus-host disease
Introduction
Pneumatosis intestinalis (PI), which is defined as gas within the bowel wall, has been reported to occur in association with many clinical conditions ranging from benign to life-threatening bowel perforation (1). PI has been reported to develop as a complication in recipients of solid organ transplantation and hematopoietic stem cell transplantation (HSCT), and the development of PI in these settings is thought to be associated with infection and immunosuppression, especially with the use of corticosteroids. In general, PI is rare after lung transplantation (LT), and to date, several cases of PI developing after LT for various lung diseases have been reported (2-10); furthermore, PI developing after LT for pulmonary graft-versus-host disease (GVHD) following HSCT has never been reported. Because HSCT recipients are high-risk candidates, owing to the prolonged immunosuppressive therapy and increased susceptibility to infections, the number of LTs performed for pulmonary GVHD developing after HSCT is still limited. In addition to the prolonged immunosuppressive therapy after HSCT, intense immunosuppression to prevent lung allograft rejection after LT might further increase the risk of PI in recipients of LT after HSCT. Herein, we describe two cases that developed PI after LT performed for pulmonary GVHD after HSCT.
Case presentation
Case 1
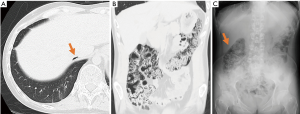
A 26-year-old man underwent bilateral cadaveric LT for pulmonary GVHD 5 years after receiving HSCT for acute myeloid leukemia (AML). Prior to the LT, the patient had never experienced gastrointestinal GVHD, either acute or chronic, and had received cyclosporine and a corticosteroid for the pulmonary GVHD. The immunosuppressive therapy after LT consisted of tacrolimus, mycophenolate mofetil and a corticosteroid. Four months after the LT, routine follow-up computed tomography (CT) showed PI involving the ascending and transverse colon, with free intraperitoneal gas (Figure 1). The patient had no symptoms, normal laboratory study results, and no evidence of any systemic or intestinal infections. He was treated conservatively without bowel rest. The PI resolved and was no longer seen on the abdominal CT performed 3 months later. At present, 20 months after the LT, the patient remains alive.
Case 2
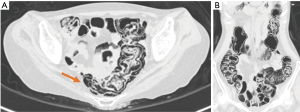
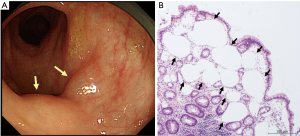
A 35-year-old woman underwent bilateral living-donor lobar LT for pulmonary GVHD developing 9 years after HSCT for Hodgkin’s lymphoma. The patient had never suffered from gastrointestinal GVHD, either acute or chronic, after the HSCT. Prior to the LT, the patient was receiving tacrolimus and a corticosteroid for pulmonary GVHD. After the LT, she received triple immunosuppressive therapy, consisting of tacrolimus, mycophenolate mofetil and a corticosteroid. The patient developed three episodes of acute rejection, necessitating steroid pulse therapy and antithymocyte globulin administration. Two months after the LT, she developed prolonged diarrhea lasting for 1 month. Laboratory examination revealed no evidence of systemic or intestinal infections, including cytomegalovirus, Clostridium difficile, or other bacterial/viral infections. Abdominal CT demonstrated PI involving the whole colon and rectum, with massive retroperitoneal air (Figure 2). Colonoscopy demonstrated multiple elevated lesions (Figure 3), and histological examination of colonoscopic biopsy specimens revealed PI without no evidence of gastrointestinal GVHD. The patient received oxygen therapy without bowel rest, and the PI disappeared within 8 days. At present, 29 months after the LT, the patient remains alive.
Discussion
To the best of our knowledge, this is the first report of PI complicating LT performed for pulmonary GVHD after HSCT. Although PI is a rarely encountered complication after LT (2-10), at our hospital, 2 of 21 recipients (9.5%) of LT after HSCT developed PI among a total of 147 recipients of LT between 1998 and 2015. Considering that the reported incidence of PI after HSCT is only 1.2–3.1% (11,12), the incidence of PI complicating LT performed after HSCT might be higher as compared to that after HSCT.
The possible causes of PI after LT include cytomegalovirus infection, Clostridium difficile colitis, corticosteroids and immunosuppressive agents (2-10). It has been considered that inflammation caused by infection destroys bowel mucosa allowing invasion of gas-producing bacteria or air itself (4). Moreover, in the reports of PI after HSCT, corticosteroids independently appear to significantly increase the risk of development of PI after HSCT (11,12). In regard to the mechanism underlying the development of PI after HSCT, it has been speculated that corticosteroids may induce atrophy of the Peyer’s patches, resulting in defects of the bowel mucosa and subsequent migration of gas or air into the submucosal and subserosal regions (11,12). Our patients showed no evidence of infection including cytomegalovirus or Clostridium difficile infection. On the other hand, they had received long-term immunosuppression with a calcineurin inhibitor and corticosteroid for 5 and 9 years, respectively, for pulmonary GVHD prior to the LT, which might have contributed to the development of the PI after the LT. After LT, high doses of corticosteroids are routinely used, while calcineurin inhibitors and mycophenolate mofetil are also used concurrently with therapeutic drug monitoring. Because the long-term immunosuppression for pulmonary GVHD might potentially damage the bowel mucosa, the intestine of patients with pulmonary GVHD might be susceptible to PI caused by the intense immunosuppression, especially administration of corticosteroids, after LT.
For the diagnosis of PI, CT is the most sensitive and specific examination (12). In the recipients of LT after HSCT, gastrointestinal GVHD should be inevitably considered in the differential diagnosis of PI. In our case 2, colonoscopic biopsy was useful to exclude gastrointestinal GVHD and diagnose PI. Because emergent surgical intervention is imperative for avoiding mortality in patients of life-threatening PI caused by bowel perforation or ischemia, PI should be carefully managed by following the treatment algorithm as described before (13). Moreover, although PI has been reported to have a high mortality rate, the mortality has been associated with surgical complications rather than with the development of peritonitis or septic shock (11,12). Both of our patients recovered conservatively, and in case 2, oxygen therapy facilitated early resolution of the PI. In addition, PI developing after LT resolved with conservative therapy alone in most of patients according to a previous report (2-10). Similar to the management of PI after HSCT, conservative management, including bowel rest, total parental nutrition and oxygen therapy, might be the first-line therapy to minimize the risk of unnecessary surgery in cases of PI developing after LT.
Frequent performance of CT after LT could increase the incidence of PI in LT recipients, especially those with a history of prolonged immunosuppression for pulmonary GVHD, and it is necessary to ensure prompt diagnosis and adequate management.
Acknowledgements
None.
Footnote
Conflicts of Interest: This work was presented at the 36th annual meeting and scientific sessions of the International Society for Heart and Lung Transplantation. April 2016; Washington, DC, USA.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Greenstein AJ, Nguyen SQ, Berlin A, et al. Pneumatosis intestinalis in adults: management, surgical indications, and risk factors for mortality. J Gastrointest Surg 2007;11:1268-74. [Crossref] [PubMed]
- Chandola R, Elhenawy A, Lien D, et al. Massive gas under diaphragm after lung transplantation: pneumatosis intestinalis simulating bowel perforation. Ann Thorac Surg 2015;99:687-9. [Crossref] [PubMed]
- Thompson WM, Ho L, Marroquin C. Pneumatosis intestinalis and pneumoperitoneum after bilateral lung transplantation in adults. AJR Am J Roentgenol 2011;196:W273-9. [Crossref] [PubMed]
- Ho LM, Mosca PJ, Thompson WM. Pneumatosis intestinalis after lung transplant. Abdom Imaging 2005;30:598-600. [Crossref] [PubMed]
- Schenk P, Madl C, Kramer L, et al. Pneumatosis intestinalis with Clostridium difficile colitis as a cause of acute abdomen after lung transplantation. Dig Dis Sci 1998;43:2455-8. [Crossref] [PubMed]
- Böhler A, Speich R, Russi EW, et al. Pneumatosis intestinalis and active cytomegaloviral infection after lung transplantation. Chest 1995;107:582-3. [Crossref] [PubMed]
- Mistrot DP, Gemma VA, Gagliano RA Jr, et al. A 54-Year-Old Man Presenting With an Abnormal Abdominal CT Scan 8 Months After Double Lung Transplant. Chest 2016;149:e151-5. [Crossref] [PubMed]
- Ling FY, Zafar AM, Angel LF, et al. Benign pneumatosis intestinalis after bilateral lung transplantation. BMJ Case Rep 2015;2015:bcr2015210701. [Crossref] [PubMed]
- Mannes GP, de Boer WJ, van der Jagt EJ, et al. Pneumatosis intestinalis and active cytomegaloviral infection after lung transplantation. Groningen Lung Transplant Group. Chest 1994;105:929-30. [Crossref] [PubMed]
- Mistrot DP, Row D, Gagliano RA, et al. Pneumatosis intestinalis in lung transplant recipients: A case series. Dis Colon Rectum 2017;60:e403.
- Hepgur M, Ahluwalia MS, Anne N, et al. Medical management of pneumatosis intestinalis in patients undergoing allogeneic blood and marrow transplantation. Bone Marrow Transplant 2011;46:876-9. [Crossref] [PubMed]
- Bhamidipati PK, Ghobadi A, Bauer S, et al. Conservative management of pneumatosis intestinalis after allogeneic hematopoietic SCT. Bone Marrow Transplant 2014;49:1436-8. [Crossref] [PubMed]
- Tahiri M, Levy J, Alzaid S, et al. An approach to pneumatosis intestinalis: Factors affecting your management. Int J Surg Case Rep 2015;6C:133-7. [Crossref] [PubMed]


