Four arms robotic-assisted pulmonary resection—right lower/middle lobectomy: how to do it
Introduction
Since its initial introduction, robotic-assisted thoracic surgery has undergone a continuous, and amazingly quick, evolution (1). Based on growing evidence of its benefits regarding the perioperative and oncological outcome, a larger number of thoracic surgeons have progressively embraced this approach for the treatment of early stage non-small cell lung cancer (NSCLC) (2). Regardless of the different techniques adopted, many authors have reported the safety and efficacy of robotically assisted lung lobectomy and highlighted the potential advantages over traditional video-assisted thoracoscopic surgery (VATS), such as magnified three-dimensional (3D) view and the endo-wrist technology that reproduce the natural movements of a surgeon’s hand (3-5). With the increasing experience and ongoing technical progress, the robotic approach has been applied to a broader range of patients including those affected by benign lung diseases (i.e., bronchiectasis, emphysema, aspergilloma). However, to date, limited data have been reported on the feasibility of robotic anatomic lung resection for those patients and further studies should be encouraged (6,7). This study aims to describe, in easy to follow, sequential steps, the four arms robotic approach, to perform right lower and middle lobectomy. We present three different cases; two patients affected by stage I, NSCLC of the middle and lower lobe respectively and one patient that presented a giant bullous emphysema of the right lower lobe, complicated by chronic infection. The detailed description of the technique is accompanied by videos of the three surgical procedures performed in two skilled institutions (Forlì Teaching Hospital and Humanitas Rozzano) and is the third paper relating to robotic VATS lobectomy atlas technique (8,9).
Clinical vignette
Case 1: robotic assisted right lower lobectomy
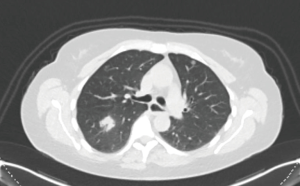
A 53-year-old lady with 20 pack-year smoking history who quit tobacco use in 2006 was admitted with a diagnosis of a 25 mm × 30 mm adenocarcinoma of the lower lobe of the right lung. Preoperative work up included whole body computer tomography (CT) and positron emission tomography-CT (PET-CT)-scan (Figure 1). The final clinical stage was cT1N0M0. The patient was scheduled for a robotic right lower lobectomy with a four-arm approach with the da Vinci Si surgical system. Overall the operative time (skin to skin) was 117 minutes. The patient was discharged on the third postoperative day (Figure 2).

Case 2: robotic assisted right middle lobectomy
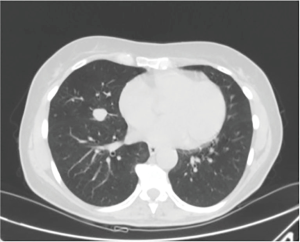
The patient was a 65-year-old lady, former smoker (30 pack-years) with no relevant medical history that was referred to our institution with an incidental discovery of a 1.7-cm well-rounded coin lesion of the middle lobe of the right lung (Figure 3). Preoperative positron emission tomography revealed standardised uptake values of 3.4. A CT guided fine needle aspiration biopsy was then performed, and a diagnosis of a carcinoid tumour was obtained. The lady underwent robotic assisted right middle lobectomy, and the final pathological stage was pT1N0M0. Surgical time was 110 minutes. The patient was discharged in a third postoperative day (Figure 4).

Case 3: robotic assisted right lower lobectomy
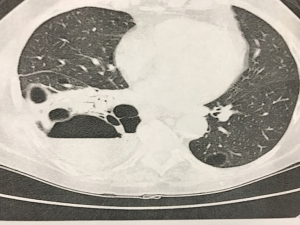
Here we presented the case of a 70-year-old lady, non-smoker, who had bullous emphysema localized to the right lower lobe, complicated by recurrent pneumonia. CT displayed an almost complete destruction of the lower lobe parenchyma that was partially replaced by a giant bulla with signs of recurrent infections (Figure 5). Due to the failure of medical/conservative treatments, the patient was considered for surgical resection. We performed a robotic right lower lobectomy in the same fashion as for lobectomy for NSCLC. The procedure required a prolonged adhesiolysis, and hilar dissection was complicated by the presence of enlarged and calcified lymph nodes. The overall surgical time was 158 minutes. The postoperative period was uneventful, and the lady was discharged on the fifth postoperative day (Figure 6).

Preference cards
- Two Cadiere forceps: the Cadiere forceps help to gently retract the lung parenchyma and to proceed with blunt dissection around vascular adventitia;
- Fenestrated Bipolar Forceps; Permanent Cautery Hook (EndoWrist Monopolar Cautery): for coagulation and dissection;
- Endowrist 8 mm clip appliers (medium-large clips): polymer vascular clips for smaller vessels;
- Endoscopic staplers: curved-tip reloads, and articulation are very useful for negotiating vessels. (Articulating Vascular/Medium Reload with Tri-Staple™ Technology, Covidien);
- Energy device: Harmonic ACE ultrasonic;
- Soft tissue retractor: AlexisTM (Applied Medical);
- Silicone vessel loops.
Surgical technique
Patient positioning & port placement
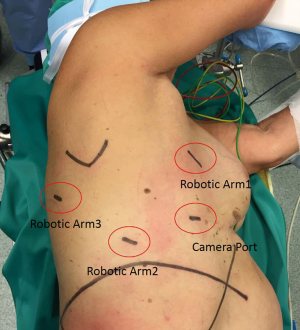
Under general anaesthesia with a double lumen intubation, the patient is placed in left lateral decubitus with the hips fixed at the level of the table break and flexed to achieve maximum separation of the intercostal spaces. The Si da Vinci robot is positioned at the head of the patient. We always proceed performing a 3-cm utility incision at the 5th intercostal space anteriorly of the latissimus dorsi. The wound is usually protected with soft tissue retractor. We then introduce a 10-mm 30-degree VATS camera to explore the chest cavity and confirm the absence of pleural effusion and metastasis. All the other ports are performed under direct view guidance. The 12-mm camera port is usually placed at the level of the midaxillary line generally at the 7th intercostal space. Two operative ports are then performed in the eighth intercostal space, 9 to 10 cm posteriorly to the camera port and the 4th port is made in the auscultatory triangle. We then start docking the robot. We always use a 30-degree stereoscopic robotic camera. Under direct view, the bed-assistant start introducing the operative robotics arms. A Cadiere forceps should be inserted through the fourth posterior trocar (arm 3), it allows to grab and retract lung parenchyma and obtain a good exposure of the lung hilum. The permanent cautery hook is then placed at the utility incision port (surgeon right hand: arm 1), and the fenestrated bipolar forceps is finally introduced operative port (surgeon right hand; arm 2) (Figure 7).
Robotic right lower lobectomy (Figure 2)
Step 1: pulmonary ligament and right lower lobe vein isolation
After inspection of the pleural surface, we proceed to divide the pulmonary ligament. After that, the lower lobe should be retracted with the Cadiere forceps (arm 3) and pulled towards the apex, exposing of the inferior pulmonary ligament. With a sponge stick introduced by the bed-assistant through the utility incision, the diaphragm dome is pushed down to improve visualization of the target area. The ligament is incised with the hook diathermy all the way up to expose the inferior edge of the lower lobe vein. The diathermy should be carefully applied to the junction between the lower lobe and the pleural reflection to avoid dissection of the parenchyma. Once the inferior pulmonary vein is visualised, the anterior mediastinal pleura is dissected to expose and identify the middle and upper lobe vein and determine the presence of a normal venous anatomy. The inferior vein is isolated and then divided with at 30-mm vascular stapler that is always introduced from the utility incision. We prefer to complete vascular dissection before dividing blood vessels.
Step 2: pulmonary artery isolation
We prefer to divide the anterior part of the oblique fissure between the right lower lobe and the middle lobe so to achieve a better exposure of the pulmonary artery in the fissure, which is then dissected proceeding with a sharp and blunt dissection. The pulmonary artery is encircled with a vessel loop and divided with at 30-mm vascular stapler that is always introduced from the utility incision. If the take-off of the superior segment branch is too separated from the basal trunk and difficult to be isolated in one single step, the artery should be divided into two separated steps.
Step 3: right lower lobe bronchus
Once the pulmonary artery is divided, the lobe should be pulled anteriorly toward the hart. Posterior mediastinal pleura is dissected, and all hilar lymph nodes should be removed. The bronchus is cut with a 60-mm stapler that we prefer to pass through the arm 2. We complete the procedure separating the posterior part of the oblique fissure. The resected lobe is placed inside a specimen bag and retrieved out of the utility incision.
Robotic right middle lobectomy (Figure 4)
To perform a four-arm robotic right middle lobectomy we prefer to proceed with the following sequence: (I) middle lobe pulmonary vein; (II) middle lobe bronchus; (III) middle lobe branch(es) of the pulmonary artery; (IV) fissure.
Step 1: right middle lobe vein isolation
The middle lobe vein is the first structure facing the surgeon. The lung is retracted posteriorly with the Cadiere forceps (arm 3, Figure 7) to expose this vessel and the anterior mediastinal pleura over the vein is opened. Dissection should be conducted along the sub-adventitial layer using a combination of sharp and blunt dissection to isolate the vein completely. Exposure of the upper and lower vein is always recommended to confirm the presence of a normal vein anatomy. The vein is then isolated and encircled with a vessel loop. To divide the middle lobe, vein the vascular stapler should be introduced from the posterior port (arm 2, Figure 7).
Step 2: right middle bronchus and oblique fissure division
Once the vein is divided, we proceed to dissect the tissue around the middle bronchus, and all adjacent hilar lymph nodes are removed. To separate the bronchus, we introduce the stapler again from the posterior port (arm 2). The oblique fissure (right middle and lower lobe fissure) is often complete; if the fissure is incomplete, we divide it introducing the stapler from the utility incision after identifying the pulmonary artery.
Step 3: pulmonary artery branch(es)
The branch for the middle lobe is determined, proceeding with a blunt dissection along the pulmonary artery in the sub-adventitia space. Usually, there is one large branch or two smaller branches. The bed-assistant should introduce the vascular stapler from the utility incision. Alternatively, in the presence of small branches, we advise dividing one branch a time placing one or two Hem-o-lok clips proximally and divide it with the harmonic device. The horizontal fissure is finally separated. Once again, the staplers are introduced from the utility incision keeping them above the underlying interlobar pulmonary artery. The lobe is then extracted through a specimen retrieval bag.
Lymph node dissection
- Station 7 (subcarinal): the lung is pulled anteriorly and towards the apex. Posterior pleura is now dissected from the superior edge of the lower lobe vein. Once the vagus nerve is identified dissection should proceed parallel to the course of the nerve until the inferior board of the azygos vein is exposed. An extensive examination of the pleura allows a better visualization of the subcarinal area;
- The paratracheal area is approached with an initial dissection of the mediastinal pleura under the azygos vein and prolonged along the lateral profile of the superior cava vein. The mediastinal pleura is then retracted posteriorly with Cadiere forceps (arm 3). Stations 2R and 4R should be removed en-bloc.
Discussion
- Performing a robotic lobectomy in the presence of a chronic inflammatory/infectious status can be more challenging, as shown in case 3. In this case, we proceeded with the usual sequential steps as for lobectomy for lung cancer. We had to deal with significant adhesions that required a prolonged complete adhesiolysis before proceeding with pulmonary resection. However, pleural adhesions do not represent per se a contraindication to robotic surgery. Once the correct pleural plane is identified, we usually deliver a combination of blunt and sharp dissection. The permanent cautery hook may speed up the procedure. In this situation, a rolled-up sponge should always be present on the field. It will help to gently retract the parenchyma that can be easily torn, particularly in the presence of giant bullous emphysema. It also contributes to keep the surgical field clean avoiding an annoying blood collecting into the chest. The 3D magnified view and precise dissection allow the surgeon to perform adhesiolysis even in the remote area of the chest;
- Care must be taken when isolating the lower lobe vein. If the dissection is too deep into the lobe, it may lead to isolation and division of only a few branches rather than the whole vein;
- Sometimes the soft tissue around the middle bronchus may offer an individual resistance and may complicate the attempt to slide the stapler around it. The lobe should be pulled anteriorly and upward to make the bronchus more vertical. The stapler should be kept parallel to the pericardium. This manoeuvre helps to pass the stapler more safely without pushing too hard;
- When the oblique fissure between the middle and lower lobe is relatively complete, then it is possible to identify the artery in the fissure quite quickly. We recommend avoiding horizontal fissure dissection (intermediate and upper lobe fissure), as it may lead to an air leak.
Conclusions
As shown above, via the operative techniques, pictures, and video the four-arm approach offers an outstanding visibility of the hilar structures during robotic surgery. The robotic four-arm approach is a feasible and reproducible method and appears to be well suited to perform a lung lobectomy safely. The utility incision (3 to 4 cm) provide enough to space to introduce one robotic arm together with the instruments controlled by the bed assistant, such as sucker, sponge stick and stapler. Moreover, in the case of uncontrolled bleeding can be converted rapidly to lateral thoracotomy. To safely find the right angle to slide the stapler around the pulmonary artery and vein we usually encircle pulmonary vessels with a vessel loop, and we prefer to use curved tip articulated vascular reload. The future of robotic surgery is promising since multiple new instruments will soon be available to make safer and simpler procedures because as reported by Leonardo da Vinci: “Simplicity is the ultimate sophistication.”
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016;101:28-34. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Toker A, Ayalp K, Uyumaz E, et al. Robotic lung segmentectomy for malignant and benign lesions. J Thorac Dis 2014;6:937-42. [PubMed]
- Gulkarov I, Ciaburri D, Tortolani A, et al. Robotic thoracoscopic resection of intralobar sequestration. J Robot Surg 2012;6:355-7. [Crossref] [PubMed]
- Pardolesi A, Bertolaccini L, Brandolini J, et al. Four arms robotic-assisted pulmonary resection-left lower lobectomy: how to do it. J Thorac Dis 2017;9:1658-62. [Crossref] [PubMed]
- Pardolesi A, Bertolaccini L, Brandolini J, et al. Four arm robotic-assisted pulmonary resection-right upper lobectomy: how to do it. J Thorac Dis 2017;9:3302-6. [Crossref] [PubMed]
- Pardolesi A, Bertolaccini L, Brandolini J, et al. Four arms robotic right lower lobectomy for NSCLC. Asvide 2018;5:051. Available online: http://www.asvide.com/article/view/22652
- Pardolesi A, Bertolaccini L, Brandolini J, et al. Four arms robotic right middle lobectomy for middle lobe carcinoid. Asvide 2015;5:052. Available online: http://www.asvide.com/article/view/22653
- Pardolesi A, Bertolaccini L, Brandolini J, et al. Four arms robotic right lower lobectomy for giant bullous emphysema localised in the right lower lobe, complicated by chronic inflammatory status. Asvide 2018;5:053. Available online: http://www.asvide.com/article/view/22654