Heart-breaking aspirin interruption
The prescription of patients with coronary artery disease is a long one: 20% take 7 drugs or more every day, and patients aged 65 years or higher often take more than 10 drugs daily. This situation reflects the current trend in research and development of new drugs usually evaluated on top of the current standard of care. The pharmaceutical industry hardly supports drug withdrawal trials, whose investigators are always Academics supported by too rare public funding (1). As a result, except for bivalirudin, few drugs or strategies have been developed to replace older ones, or reduce the duration and number of treatments. Along with adverse events and socio-economic level, this increased number of life-long treatments on prescription is one of the reasons for poor compliance and drug discontinuation that occur in 20% to 40% of patients. More alarming, high risk patients seem to have the lowest observance, especially among active smokers and diabetic patients, with important ischemic and economic consequences (2). Studies evaluating interruption of aspirin demonstrated that what is true for the heart is also valid for the brain: it leads to a significant increase of recurrent myocardial infarction, ischemic stroke and cardiovascular death. However, the risk of spontaneous aspirin discontinuation after a long term use was not well documented before the study of Sundström et al. published in recent issue of Circulation (3).
In this Swedish Nationwide population-based cohort study, investigators analyzed 601,527 aspirin long-time users and compared ischemic outcomes of patients who discontinued aspirin after 1 year or more treatment with patients who continued the drug (3). Patients with prior bleeding or surgery were excluded and their events were not reported. In the cohort, 15% of patients spontaneously discontinued aspirin after at least a year treatment. These patients suffered a 37% increase of cardiovascular events. This corresponds to one additional cardiovascular event for every 74 patients who discontinued aspirin each year. Discontinuation in secondary prevention setting led to an increase of 46% of cardiovascular events, while discontinuation in patients taking aspirin for primary prevention was associated with a 28% increase of cardiovascular events.
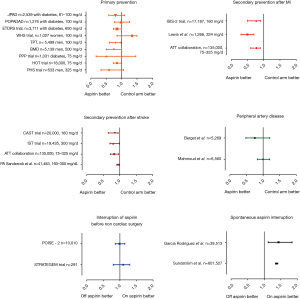
We can speculate that patients who discontinued aspirin had the heaviest prescription and the highest thrombotic burden compared to patients with good compliance. Unfortunately, we have limited information on cardiovascular risk factors, prior medical history or prescription details in this study. Interestingly, while aspirin is not recommended in primary prevention by the international guidelines, the debate is ongoing in the scientific community. This real-world registry indirectly suggests that primary prevention, when considered necessary by physicians, is effective since aspirin interruption led to disease activation with the occurrence of ischemic events. However, it is likely that the highest risk patients were selected for primary prevention with aspirin, in contrast to many primary prevention trials which constantly failed to reduce cardiovascular events in several groups of patients, including diabetics (4,5) (Figure 1). Still, the hypothesis is currently being assessed in the ARRIVE (Aspirin to Reduce Risk of Initial Vascular Events, NCT00501059) and ASCEND (A Study of Cardiovascular Events in Diabetes, NCT00135226) randomized trials, including patients with moderate risk and diabetes, respectively.
The hike of ischemic events at aspirin interruption seems to correspond to a rebound effect also documented in interruption studies focusing on ischemic cerebral stroke (18). This could result from a rebound in platelet reactivity due to increasing thromboxane levels. The early increase of ischemic events occurring in the Swedish study after aspirin discontinuation adds support to this plausible biological rebound. The absence of excess ischemic risk in patients who discontinued aspirin while on anticoagulant or another antiplatelet agent supports the idea of not removing the last antithrombotic treatment in coronary patients.
Studies assessing passive or active discontinuation of drugs targeting atherothrombosis after a long period of prescription are important. First, they reassure both physicians and patients about the benefit and safety of a life-long prescription of drugs such as aspirin, and guide them within the questioning caused by possible adverse events. For instance, interruption studies showed that keeping aspirin after an acute peptic bleeding ulcer was safe, while withdrawal led to a significant increase of mortality (19). Second, interruption studies allow a re-assessment of therapies remotely from the index event, in specific subgroups of patients or particular setting of illness that clinical trials could not evaluate. The case of statins is an interesting one: while its interruption led to an increased risk of myocardial infarctions and ischemic events in the general patient population, removing them from prescriptions of patients with an advanced illness was safe, improved the quality of life and diminished medical costs (20). Third, interruption studies can demonstrate the futility of a long-term treatment once the acute benefit is obtained: this could be the case of beta-blockers frequently renewed after an uncomplicated myocardial infarction (21). This is the current hypothesis of the ABYSS trial (Assessment of Beta blocker interruption after uncomplicated myocardial infarction on safety and symptomatic cardiac events requiring hospitalization) evaluating interruption of beta blockers 6 months after an uncomplicated myocardial infarction.
Since the Elwood et al. randomized trial in 1974 and the meta-analysis of the antithrombotic realists collaboration in 2002 that demonstrated the benefits of aspirin in secondary prevention, its place in the prescription of ischemic patients is challenged (22,23). The results of Sundström et al. are double edged for aspirin: while its interruption is harmful, patients on another antiplatelet or anticoagulant have a low risk of ischemic events when aspirin is discontinued. This supports current guidelines for the eviction of aspirin for patients with stable coronary artery disease when they are anticoagulated for a concomitant atrial fibrillation. This is consistent with the results of the recent major trials evaluating triple antithrombotic therapy, all promoting an early removal of aspirin in this type of patients (2,24,25). Regarding the ischemic hike described in the study after aspirin interruption, it will be interesting to see if early discontinuation for ticagrelor monotherapy is safe after PCI: this strategy is currently being assessed in the ongoing randomized TWILIGHT (Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention, NCT02270242) and GLOBAL LEADERS (NCT01813435) trials.
Long-term users of aspirin have other reasons to continue their treatment: oncologists have been taking interest in aspirin for primary prevention of cancer. Several cohort studies established an association with aspirin long-term treatment and decrease of cancer risk, especially colorectal cancer. In a meta-analysis comparing aspirin to placebo in 14,033 patients, we observed at decrease of 20% in colorectal cancer relative risk at 20 years (26). Four randomized trials are currently evaluating aspirin in primary prevention of cancer, secondary prevention of recurrences or adjuvant therapy (NCT00565708, NCT02394769, NCT02467582, NCT00002527). Another potential important benefit of long-term aspirin treatment could be the prevention of cognitive decline and neurodegenerative processes, through the anti-inflammatory effect of cyclooxygenase 2 inhibition in brain tissue. This hypothesis is being evaluated in the ASPREE trial (Aspirin in Reducing Events in the Elderly, NCT01038583).
In conclusion this study brings an important answer to the frequently asked question “Can I stop aspirin?” and concretely highlights the importance of maintaining adherence to chronic aspirin therapy. Despite the evolution of strategies involving direct oral anticoagulants and strong P2Y12 inhibitors, aspirin remains a corner stone of secondary prevention. Primary prevention against cardiovascular diseases, but also cancer and cognitive decline, might help aspirin to continue for another century of prevention.
Acknowledgements
None.
Footnote
Conflicts of Interest: G Montalescot has received research grants or honorarium from ADIR, Amgen, AstraZeneca, Bayer, Berlin Chimie AG, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, Cardiovascular Research Foundation, Celladon, CME Resources, Daiichi-Sankyo, Eli-Lilly, Europa, Elsevier, Fédération Française de Cardiologie, Fondazione Anna Maria Sechi per il Cuore, Gilead, ICAN, Janssen, Lead-Up, Menarini, Medtronic, MSD, Pfizer, Sanofi-Aventis, Servier, The Medicines Company, TIMI Study Group, WebMD. M Zeitouni has no conflicts of interest to declare.
References
- Dewilde WJ, Oirbans T, Verheugt FW, et al. WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107-15. [Crossref] [PubMed]
- Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013;382:1714-22. [Crossref] [PubMed]
- Sundström J, Hedberg J, Thuresson M, et al. Low-Dose Aspirin Discontinuation and Risk of Cardiovascular Events A Swedish Nationwide, Population-Based Cohort Study. Circulation 2017;136:1183-92. [Crossref] [PubMed]
- Saito Y, Okada S, Ogawa H, et al. The long-term therapy with low-dose aspirin did not reduce cardiovascular events in patients with type 2 diabetes in primary prevention: 10-year follow-up of a randomized controlled trial. Circulation 2017;135:659-70. [Crossref] [PubMed]
- Belch J, MacCuish A, Campbell I, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008;337:a1840. [Crossref] [PubMed]
- Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293-304. [Crossref] [PubMed]
- Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council's General Practice Research Framework. Lancet 1998;351:233-41. [Crossref] [PubMed]
- Sacco M, Pellegrini F, Roncaglioni MC, et al. PPP Collaborative Group Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care 2003;26:3264-72. [Crossref] [PubMed]
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-62. [Crossref] [PubMed]
- Lewis HD Jr, Davis JW, Archibald DG, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med 1983;309:396-403. [Crossref] [PubMed]
- Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988;2:349-60. [PubMed]
- CAST. randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997;349:1641-9. [Crossref] [PubMed]
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997;349:1569-81. [Crossref] [PubMed]
- Sandercock PA, Counsell C, Gubitz GJ, et al. Antiplatelet therapy for acute ischaemic stroke Cochrane Database Syst Rev 2008.CD000029. [PubMed]
- Berger JS, Krantz MJ, Kittelson JM, et al. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA 2009;301:1909-19. [Crossref] [PubMed]
- Mahmoud AN, Elgendy AY, Rambarat C, et al. Efficacy and safety of aspirin in patients with peripheral vascular disease: An updated systematic review and meta-analysis of randomized controlled trials. PLoS One 2017;12:e0175283. [Crossref] [PubMed]
- Eikelboom JW, Kearon C, Guyatt G, et al. Perioperative Aspirin for Prevention of Venous Thromboembolism: The PeriOperative ISchemia Evaluation-2 Trial and a Pooled Analysis of the Randomized Trials. Anesthesiology 2016;125:1121-9. [Crossref] [PubMed]
- García Rodríguez LA, Cea Soriano L, Hill C, et al. Increased risk of stroke after discontinuation of acetylsalicylic acid: a UK primary care study. Neurology 2011;76:740-6. [Crossref] [PubMed]
- Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med 2010;152:1-9. [Crossref] [PubMed]
- Kutner JS, Blatchford PJ, Taylor DH, et al. Safety and Benefit of Discontinuing Statin Therapy in the Setting of Advanced, Life-LimitingIllness: A Randomized Clinical Trial. JAMA Intern Med 2015;175:691-700. [Crossref] [PubMed]
- Dondo TB, Hall M, West RM, et al. Gale CP β-Blockers and Mortality After Acute Myocardial Infarction in Patients Without Heart Failure or Ventricular Dysfunction. J Am Coll Cardiol 2017;69:2710-20. [Crossref] [PubMed]
- Elwood PC, Cochrane AL, Burr ML, et al. A randomised controlled trial of acetyl salicylic acid in the secondary prevention of mortality from myocardial infarction. Br Med J 1974;1:436-40. [Crossref] [PubMed]
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86. [Crossref] [PubMed]
- Gibson CM, Mehran R, Bode C, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI N Engl J Med 2016;375:2423-34. [Crossref] [PubMed]
- Cannon CP, Bhatt DL, Oldgren J, et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med 2017;377:1513-24. [Crossref] [PubMed]
- Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741-50. [Crossref] [PubMed]