Lung volume reduction with endobronchial coils for patients with emphysema
Background
Emphysema is characterized by lung parenchymal destruction caused by tobacco smoking, inhalation of other toxic agents, together with predisposed genetic host factors such as α1-antitrypsin deficiency (1). Lung parenchymal tissue destruction in severe emphysema is associated with increased lung elasticity, loss of elastic recoil, expiratory airway collapse, leading to static as well as dynamic hyperinflation and causing a significant reduction of lung function, exercise capacity and quality of life.
For patients with severe emphysema, the current available treatment options are: smoking cessation, bronchodilators, anti-inflammatory agents, vaccinations, proper nutrition, pulmonary rehabilitation, the use of oxygen, chronic non-invasive ventilatory support and surgical interventions like lung volume reduction surgery and lung transplantation. Despite all these available treatment options, the majority of patients still remains highly symptomatic or do not qualify for surgical techniques.
Several minimal invasive bronchoscopic treatment options for severe emphysema have emerged, such as endobronchial valves (2), lung volume reduction coils (3) and more experimental techniques such as bronchoscopic thermal vapor ablation (4) and biological lung volume reduction (Aeriseal lung sealant) treatment (5), all aiming at reducing hyperinflation (6). Also very new airway directed treatments such as targeted lung denervation (7) and metered liquid nitrogen cryospray (8) are in development. Hyperinflation is known to play a key role in the feelings of dyspnea and reduced exercise capacity in emphysema (9,10). Targeting this hyperinflation component might significantly relief dyspnea and increase quality of life and exercise performance (2,11).
Depending on appropriate patient selection and correct placement, endobronchial valves reduce hyperinflation which manifests in clinical improvement (12). Responders to valve therapy are only patients with absence of interlobar collateral flow (assessed by quantitative CT fissure analysis, and/or the CHARTIS® catheter system) between the target lobe and adjacent lobe (2,13,14).
For patients with presence of interlobar collateral ventilation, of which prevalence is estimated to be around 60% in severe emphysema (15), coils might be a potential treatment option (16).
Lung volume reduction with coils
The coil
The RePneu® coil treatment (RePneu® coil system, PneumRx Inc./BTG, Santa Clara, CA, USA) is a bronchoscopic therapy for the treatment of patients with severe emphysema. The coil consists of a nickel-titanium alloy (nitinol) which exhibits a shape memory effect and is biologically inert (Figure 1). The first application in humans was performed in 2008 after extensive testing of the treatment in animal models (17). The coil is produced in three different sizes (100/125/150 mm) to accommodate different airway lengths.
Treatment procedure
The procedure is preferably performed with the patient undergoing general anesthesia, using a 9 mm flexible endotracheal tube with pressure controlled ventilation at a low ventilation frequency (~10/min) with an inspiratory/expiratory ratio of about 1:4 to allow sufficient expiration in these severely air-trapped patients. Normally, patients remain hospitalized one night for regular observation after treatment. All our patients receive both corticosteroids (prednisolone 30 mg per day), from the pre-treatment day up to 5 days after treatment, as well as antibiotic prophylaxis (azithromycin 250 mg per day) starting on the treatment day up to 30 days post treatment (expert opinion).
The coil placement procedure is, for safety reasons, performed in two separate treatment sessions, targeting one lobe per session, the contralateral lobe being treated 4 to 8 weeks after the first session. Bilateral treatment is necessary to achieve optimal treatment benefit (3). The most diseased lobes should be treated, identified using quantitative CT analysis and when needed perfusion scanning as guidance. Coil placement is performed using a bronchoscope with a therapeutic size working channel (2.8 mm internal diameter or larger). It is recommended to take a routine microbacterial culture sample during the first inspection of the bronchial tree, this to be optimally informed about airway colonization, with respect to potential future infectious events.
The coils are delivered, bronchoscopically, into the segmental and subsegmental airways using a special catheter delivery system. Placement is performed under fluoroscopy to visualize positioning and coil sizing (Figure 2). The procedure starts with a guidewire, bearing fluoroscopic markers, that is used to measure airway length and to position the coil at a fair distance from the pleura (to avoid pneumothorax and pleural pain). When the guidewire is in the correct position, a delivery catheter can be advanced over the guidewire. The coils are situated in this delivery catheter in a straight configuration. When the target treatment area is reached, the delivery catheter is pulled back and the coil reverts to its non-straightened coil shape, resulting in a compression of the local lung parenchyma. The coil can then subsequently be released.
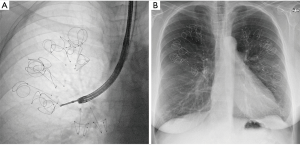
In one treatment session, around 10 to 12 coils for upper lobes and 10 to 14 coils for lower lobes are being placed in the desired lobe. During the procedure the coils can be removed and repositioned. The coil treatment is regarded permanent. However, when for example persistent thoracic pain requires removal of one coil, this has been shown feasible up to 10 months after implantation in specialist centers (18).
Mechanism of action
The hypothesized mechanism of action of the coil treatment is that the compression of the lung parenchyma by the coils results in less hyperinflation and simultaneously better transmits the elastic recoil pressure, meaning a real lung volume reduction effect (19). Secondly, the coils reduce airflow towards the targeted segments of the lung and this consequently results in a redistribution of airflow towards healthier parts of the lung (20). Furthermore, a decrease in airway resistance occurs in the treated lobes (19,21). Finally, the volume reduction of the emphysematous treated areas could improve lung compliance and put the diaphragm in a better condition of function with, as a consequence, an increase in driving pressure of the expiratory flows (19,22,23).
Feasibility & efficacy
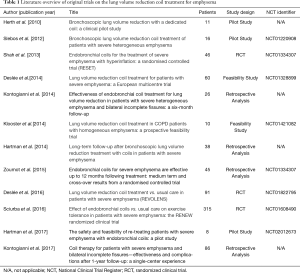
An overview of all published original coil studies is presented in Table 1.
Full table
The first pilot study on coil treatment started in 2008 in Heidelberg (Germany). Eleven patients were treated with up to 6 coils per lobe, demonstrating both feasibility and safety, but no statement on efficacy could be made (17).
The second pilot study started in 2009 in Groningen (The Netherlands). Sixteen patients were treated, demonstrating safety, feasibility and efficacy of the procedure by using the second generation of the coil and increasing the number of coils per treated lobe to 10–12. At 6-month follow-up after the final treatment, there were significant improvements of −14.9 points (P<0.001) in St. George’s Respiratory Questionnaire (SGRQ), −11.4% (P<0.001) in residual volume (RV), +84.4 meter (P<0.001) in 6-minute walking distance (6MWD) and +14.9% (P=0.004) in forced expiratory volume in 1 second (FEV1), compared to baseline (24).
The third study and first randomized controlled trial investigating coils was the RESET trial (Endobronchial coils for the treatment of severe emphysema with hyperinflation). Forty-six patients with both homogeneous and heterogeneous emphysema were allocated in a one-to-one ratio to either coil treatment (treatment group) or best medical care (control group). Patients were treated in two sessions, with the contralateral lobe being treated 1 month after the initial treatment. Outcome measures were performed 90 days after the final treatment or the equivalent visit for the usual care group. Differences between treatment and best medical care group scores in change from baseline were −8.4 points (P=0.04) in SGRQ, −0.31 L (P=0.03) in RV, +63.6 meter (P<0.001) in 6MWD and +10.6% (P=0.03) in FEV1 at 90 days follow-up after the final treatment (25).
The fourth study, an open label feasibility study, investigating coils in strict homogeneous emphysema, confirmed the efficacy of treatment for this phenotype. At 6 months follow-up after treatment, there were significant improvements of −15 points (P=0.028) in SGRQ, −0.6 L (P=0.007) in RV, +61 meter (P=0.005) in 6MWD and +18.9% (not significant, P=0.102) in FEV1, compared to baseline (21).
The fifth study, a European open-label feasibility study including 60 patients, confirmed the previously published single center results in a multicenter design with a good safety profile and sustained results up to 12 months follow-up. At 12 months follow-up after treatment, there were significant improvements of −11.1 points (P<0.001) in SGRQ, −0.71 L (P<0.001) in RV, +51.4 meter (P=0.003) in 6MWD and 0.11 L (P=0.037) increase in FEV1, compared to baseline (26).
The sixth study and second randomized controlled trial was the REVOLENS trial (Lung Volume Reduction Coil Treatment vs. Usual Care in Patients with Severe Emphysema). One hundred patients were allocated in a one-to-one ratio to either coil treatment or usual care. Contralateral treatment took place 1 to 3 months after the first. Approximately 10 coils per targeted lobe were delivered. All patients were assessed at baseline and at 1, 3, 6 and 12 months after baseline. Differences between treatment and usual care group scores in change from baseline were −13.4 points (P<0.001) in SGRQ, −0.37 L (P=0.01) in RV, +21 meter (not significant, P=0.06) in 6MWD and +11% (P=0.01) in FEV1 at 6 months post treatment (27).
The seventh study and third randomized controlled trial was the RENEW trial (Effect of Endobronchial Coils vs. Usual Care on Exercise Tolerance in Patients with Severe Emphysema), including 315 patients. Differences between treatment and usual care group scores in change from baseline were −8.9 points (P<0.001) in SGRQ, −0.31 L (P=0.01) in RV, +14.6 meter (P=0.02) in 6MWD and +7% (P<0.01) adjusted median increase in FEV1 at 12 months post treatment. The greatest improvements occurred in the RV ≥225% predicted subgroups, in both heterogeneous and homogeneous emphysema phenotypes, highlighting the importance of the presence of hyperinflation (11).
An overview of efficacy outcomes of the larger studies is provided in Table 2.

Full table
Safety-profile
The most common complications of coil treatment are: chronic obstructive pulmonary disease (COPD) exacerbations, pneumonia, Coil Associated Opacity and an increased risk of pneumothorax (11,25,27).
In a 2015 meta-analysis, including 140 patients, no serious adverse events occurred periprocedural in any of the 259 coil procedures and no deaths or respiratory failures were reported. A total of 37 severe COPD exacerbations and 27 pneumonias requiring hospitalization were recorded among all patients up to 1 year of follow-up. Pneumothorax occurrence for which chest tube insertion was required was 6.4% per patient treated. Severe COPD exacerbation incidence was 3.1% in the first month after treatment, 2.9% per month from 1 to 6 months after treatment and 2.3% per month from 6 months up to 1 year follow-up. Pneumonia incidence was 3.5% per month during the first month after treatment, 1% from 1 to 6 months after treatment and 2.1% per month from 6 months up to 1 year follow-up (3).
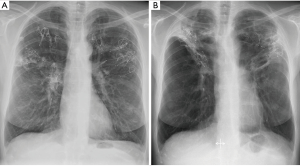
Coil Associated Opacity, a phenomenon first described by the “RENEW” study investigators, is a noninfectious, localized tissue response that occurs post-coil implantation in approximately 5–10% of cases. Coil Associated Opacity is hypothesized to be induced by stress forces from the coils on lung parenchyma. Patients with Coil Associated Opacity can demonstrate symptoms comparable to infectious pneumonia and this makes it difficult to distinguish between them. A chest radiograph of a patient with Coil Associated Opacity is provided in Figure 3. Patients with Coil Associated Opacity exhibited superior 12-month effectiveness outcomes compared to patients without Coil Associated Opacity or pneumonia (11).
Patient selection criteria
Coils are a potential treatment option for patients who do not qualify for endobronchial valve treatment [due to for example positive interlobar collateral ventilation status (16)] or lung volume reduction surgery, and can also be offered as a bridge to lung transplantation. Selecting optimally treated, symptomatic COPD patients with emphysema and severe hyperinflation (absolute minimal criteria for hyperinflation: RV >200% predicted and RV/TLC ratio >58%, measured using body plethysmography), while avoiding significant airway disease such as asthma, chronic bronchitis and bronchiectasis, is key to achieve treatment success (12,28,29). Additional patient inclusion and exclusion criteria specific for the coil treatment from our center are summarized in Table 3.

Full table
Long term follow-up & re-treatment with coils
To date, not a lot of data exists on longer term outcome after coil treatment. One single center study investigated the safety and efficacy of the coil treatment in the long term at 1, 2 and 3 years follow-up. At 3-year follow-up, no long-term unexpected adverse and device-related events occurred, with clinical benefit gradually declining over time (30).
Re-treatment with coils has been investigated in one pilot study, including eight patients. Re-treatment was performed at a median of 1,382 days after initial coil treatment with a median additional of 12 coils per patient. The trail was not powered for efficacy outcomes. No unexpected adverse events occurred, suggesting feasibility and safety of re-treatment (31).
Cost-effectiveness
Cost-effectiveness of the coil treatment has been investigated in the REVOLENS trial. Cost was estimated at $47,908 per patient above usual care at 1 year and the incremental cost-effectiveness ratio was $782,598 per additional quality-adjusted life-year. However, the short duration of the follow-up prevented the authors from drawing a conclusion on long term cost-effectiveness, as the financial costs of procedure and devices should be allocated over the total duration of clinical benefit. Possibly, the expected 5-year follow-up data from this clinical trial will provide more insight in cost-effectiveness of the coil treatment (27).
Conclusions and future perspectives
Three randomized clinical trials investigating coil treatment have been published until now, reporting the results of 452 treated patients up to 12 months after coil treatment. In these trials, the coil treatment results in significant improvements in pulmonary function and especially quality of life in patients with severe hyperinflation.
Since treatment can be performed regardless of collateral ventilation status it may be an effective treatment for patients who are not eligible for endobronchial valve treatment or other collateral ventilation dependent interventions. In addition, both patients with a homogeneous and heterogeneous phenotype can be treated. The selection of optimally treated, symptomatic COPD patients with severe emphysema and severe hyperinflation while avoiding significant airway disease such as asthma, chronic bronchitis and bronchiectasis, is key to achieve treatment success.
Several new studies are currently underway: the first one being the “REACTION study: Identifying Responders and Exploring Mechanisms of ACTION of the Endobronchial Coil Treatment for Emphysema” (www.clinicaltrials.gov identifier: NCT02179125), a non-randomised open label multi-center intervention study. The objectives are to gain more knowledge on the mechanism of action, identifying predictors of response and describing the effect on patient-based outcomes of endobronchial coil treatment.
A post-marketing study titled “Changes in Lung Physiology and Cardiac Performance in Patients with Emphysema Post Bilateral RePneu Coil Treatment” (NCT02499380) is aimed at understanding the mechanism of action of the RePneu coil by observing changes in lung physiology and cardiac performance in patients treated with RePneu coils.
Another study: “LVRC-Micro: Lung Volume Reduction Coil Microbiome Study” (NCT03010566), aims to investigate possible changes in the microbiome of the lungs in patients 6 months after initial coil treatment.
An overview of current ongoing studies on coil treatment can be found in Table 4.

Full table
Future research is necessary to provide more insight in different aspects of the coil treatment. Whilst studies investigating the mechanism of action of the intervention and predictors of response are underway, more work is needed to refine patient selection, assess durability of treatment benefit and determine long term cost-effectiveness.
Acknowledgements
None.
Footnote
Conflicts of Interest: JBW declares no conflict of interest. DJS is a physician advisor to PneumRx/BTG, is a principal investigator for trials sponsored by PneumRx/BTG, and received lecture and travel fees for case support, educational and scientific sessions.
References
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. [Crossref] [PubMed]
- Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial Valves for Emphysema without Interlobar Collateral Ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Slebos DJ, Hartman JE, Klooster K, et al. Bronchoscopic Coil Treatment for Patients with Severe Emphysema: A Meta-Analysis. Respiration 2015;90:136-45. [Crossref] [PubMed]
- Herth FJ, Valipour A, Shah PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6-month results of the multicentre, parallel-group, open-label, randomised controlled STEP-UP trial. Lancet Respir Med 2016;4:185-93. [Crossref] [PubMed]
- Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651-62. [Crossref] [PubMed]
- Mineshita M, Slebos DJ. Bronchoscopic interventions for chronic obstructive pulmonary disease. Respirology 2014;19:1126-37. [Crossref] [PubMed]
- Slebos DJ, Klooster K, Koegelenberg CF, et al. Targeted lung denervation for moderate to severe COPD: a pilot study. Thorax 2015;70:411-9. [Crossref] [PubMed]
- Slebos DJ, Breen D, Coad J, et al. Safety and Histological Effect of Liquid Nitrogen Metered Spray Cryotherapy in the Lung. Am J Respir Crit Care Med 2017;196:1351-2. [Crossref] [PubMed]
- Cooper CB. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006;119:21-31. [Crossref] [PubMed]
- O’Donnell DE, Laveneziana P. Dyspnea and activity limitation in COPD: mechanical factors. COPD 2007;4:225-36. [Crossref] [PubMed]
- Sciurba FC, Criner GJ, Strange C, et al. Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. JAMA 2016;315:2178-89. [Crossref] [PubMed]
- Herth FJF, Slebos DJ, Criner GJ, et al. Endoscopic Lung Volume Reduction: An Expert Panel Recommendation - Update 2017. Respiration 2017;94:380-8. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]
- Kemp SV, Slebos DJ, Kirk A, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. [Crossref] [PubMed]
- Koster TD, Slebos DJ. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. Int J Chron Obstruct Pulmon Dis 2016;11:765-73. [Crossref] [PubMed]
- Kontogianni K, Gerovasili V, Gompelmann D, et al. Effectiveness of endobronchial coil treatment for lung volume reduction in patients with severe heterogeneous emphysema and bilateral incomplete fissures: a six-month follow-up. Respiration 2014;88:52-60. [Crossref] [PubMed]
- Herth FJ, Eberhard R, Gompelmann D, et al. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis 2010;4:225-31. [Crossref] [PubMed]
- Dutau H, Bourru D, Guinde J, et al. Successful Late Removal of Endobronchial Coils. Chest 2016;150:e143-e5. [Crossref] [PubMed]
- Palamidas AF, Kemp SV, Shen M, et al. Putative Mechanisms of Action of Endobronchial Coils. Am J Respir Crit Care Med 2017;196:109-15. [Crossref] [PubMed]
- Kloth C, Thaiss WM, Hetzel J, et al. Impact of endobronchial coiling on segmental bronchial lumen in treated and untreated lung lobes: Correlation with changes in lung volume, clinical and pulmonary function tests. Eur Radiol 2016;26:2176-83. [Crossref] [PubMed]
- Klooster K, Ten Hacken NH, Franz I, et al. Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration 2014;88:116-25. [Crossref] [PubMed]
- Fessler HE, Scharf SM, Ingenito EP, et al. Physiologic basis for improved pulmonary function after lung volume reduction. Proc Am Thorac Soc 2008;5:416-20. [Crossref] [PubMed]
- Ingenito EP, Loring SH, Moy ML, et al. Interpreting improvement in expiratory flows after lung volume reduction surgery in terms of flow limitation theory. Am J Respir Crit Care Med 2001;163:1074-80. [Crossref] [PubMed]
- Slebos DJ, Klooster K, Ernst A, et al. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 2012;142:574-82. [Crossref] [PubMed]
- Shah PL, Zoumot Z, Singh S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med 2013;1:233-40. [Crossref] [PubMed]
- Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicentre trial. Thorax 2014;69:980-6. [Crossref] [PubMed]
- Deslée G, Mal H, Dutau H, et al. Lung Volume Reduction Coil Treatment vs Usual Care in Patients With Severe Emphysema: The REVOLENS Randomized Clinical Trial. JAMA 2016;315:175-84. [Crossref] [PubMed]
- Hartman JE, Klooster K, Ten Hacken NH, et al. Treatment of emphysema using bronchoscopic lung volume reduction coil technology: an update on efficacy and safety. Ther Adv Respir Dis 2015;9:251-9. [Crossref] [PubMed]
- Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial Valves for Endoscopic Lung Volume Reduction: Best Practice Recommendations from Expert Panel on Endoscopic Lung Volume Reduction. Respiration 2017;93:138-50. [Crossref] [PubMed]
- Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015;20:319-26. [Crossref] [PubMed]
- Hartman JE, Klooster K, Ten Hacken NHT, et al. The Safety and Feasibility of Re-treating Patients with Severe Emphysema with Endobronchial Coils: A Pilot Study. COPD 2017;14:339-43. [Crossref] [PubMed]



