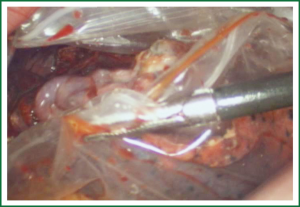
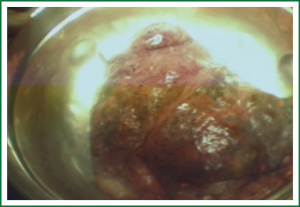
Unidirectionally progressive left pneumonectomy & mediastinal lymph node dissection
Introduction
In recent years, video-assisted thoraco-scopic surgery (VATS) has been rapidly developing (1). With smaller incisions, ease to operate, minimal invasiveness, avoidance of resecting the ribs, faster opening and closure of the chest and reduced intraoperative blood loss, this technique allows lower postoperative pain of the incision area and reduced incidence of venous thromboembolism (2). Thus, it has been widely applied in mediastinal tumor resection, wedge resection of lung masses, lobectomy, radical treatment of lung cancer, excavation of esophageal leiomyomas, esophageal tumor resection and other procedures. In particular, thoracoscopic lobectomy has become the standard surgical approach for treating lung cancer recommended by the National Comprehensive Cancer Network and the American Association of Chest Physicians in 2006 and 2007. However, few reports have described the application of complete video-assisted techniques in left pneumonectomy. Small-incision thoracoscopic complete left pneumonectomy, which has been conducted in several hospitals, differs specifically from its complete video-assisted counterpart in that it does not require thoracic distraction of the intercostal space, and the operation is not performed through visual observation via the incision. Hence, the postoperative wound pain is significantly improved. Excessive reliance on the direct observation through a small incision has deprived the technological advantages of thoracoscope in almost all aspects, making it a mere “premium light source”. Compared with conventional thoracotomy and small incision thoracoscopic surgery, this technique is advantageous in its less injury, reduced pain, fewer complications, and faster recovery (3,4). In general, chemotherapy and radiotherapy can be performed in 3-4 weeks after the surgery, with comparable effect to conventional thoracotomy. At present, thoracoscopic lobectomy is mainly performed in the traditional or unidirectionally progressive ways. The former was established by Jianxing He in 1994, who was a pioneer in applying this approach in China. In traditional lobectomy, the lobectomy is completed by handling the pulmonary fissure, the pulmonary artery, the pulmonary vein, and the bronchus in this order. Flipping of the lung lobes is often required. The single-direction concept was first introduced by Liu et al. (5) in China and applied in clinical treatment, which eliminated the need for flipping the lobes back and forth, up and down. The surgical resection is generally completed unidirectionally from the anterior to posterior regions when removing the upper or middle lobes, or from the inferior to superior regions when dealing with the lower lobe. In view of the specific structure of the pulmonary hilum, the operation is progressed in the order of freeing and separating the pulmonary vein, the bronchus, the pulmonary artery, and the pulmonary fissure. In this video (Video 1), unidirectionally progressive left pneumonectomy is performed in the order of left superior pulmonary vein—left lower pulmonary vein—left main bronchus—left pulmonary artery.
Case report
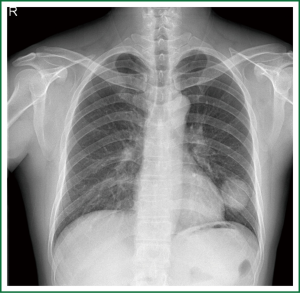
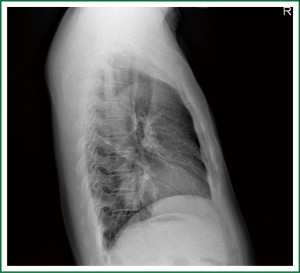
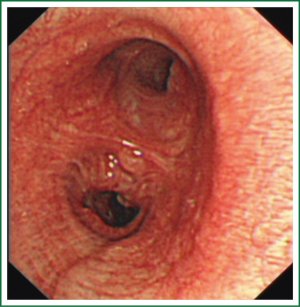
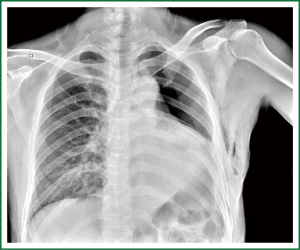
This case is a 48-year-old male patient admitted for irritating cough for two weeks. Before hospitalization, enhanced chest CT had been conducted at a local hospital, which suggested left lower lobe tumor about the size of 46 mm × 39 mm. A repeat chest anteroposterior radiograph was conducted (Figures 1,2). Bronchoscopy showed a neoplasm in the opening of the left upper lobe, and scattered masses at the opening of the left lower lobe (Figure 3). Biopsy suggested moderately differentiated adenocarcinoma of the left upper lung and poorly differentiated adenocarcinoma of the left lower lung. Following head MRI, ECT body bone scintigraphy, ultrasound of abdominal organs and other related auxiliary examination, no distant metastases were found. Based on the preoperative laboratory examinations, a final decision was made to perform complete unidirectionally progressive left pneumonectomy + mediastinal lymph node dissection. The resecting order was left superior pulmonary vein—left lower pulmonary vein—left main bronchus—left pulmonary artery. Pathology of the resected left lung showed differentiated adenocarcinoma, consistent with the preoperative diagnosis. Following intraoperative dissection of mediastinal lymph node groups 5, 6, 9 and group 10 hilar lymph nodes, metastases were found in all specimens. The complete thoracoscopic surgery resulted in small incisions and faster recovery. After removal of the chest tube, no abnormal finding was present on chest radiographs (Figure 4).
Video description
Challenging steps
Since the patient is treated with VATS lobectomy, the key to success is similar to traditional lobectomy, which includes thorough lymph node dissection, and proper management of major intraoperative bleeding.
The surgery is performed under intravenous general anesthesia using double-lumen endotracheal intubation, with contralateral one-lung ventilation. The double-lumen tube intubation is essential for VATS surgery because it is mandatory to collapse the lung on the side of thoracoscopic surgery to allow smooth access.
The patient is placed in a 90-degree position lying on the unaffected side.
Incision
Similar to traditional resection of left lung lobes, an approximately 1.5-cm observation port is created in the 7th intercostal space between the middle and anterior axillary lines, an approximately 4-cm working port in the 4th intercostal space between the anterior axillary line and the midclavicular line, and an approximately 1.5-cm auxiliary port in the 9th intercostal space between the posterior axillary line and the subscapular line. The operator stands in front of the patient, manipulating the endoscopic instruments while watching the monitor.
Surgical sequence
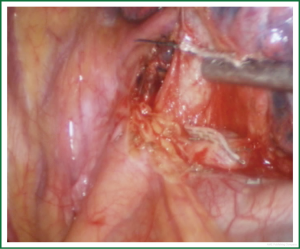
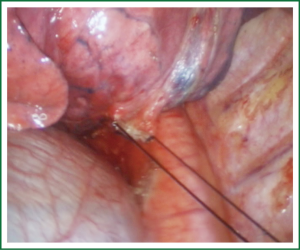
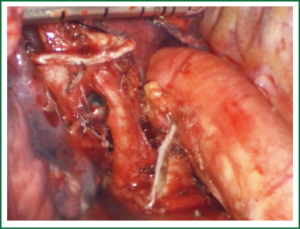
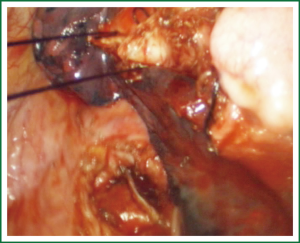
For traditional lobectomy, the following structures are operated in this order: (I) the right upper lobe: pulmonary vein—pulmonary artery—horizontal fissure—posterior ascending branch of the pulmonary artery—poorly developed fissure—bronchus; (II) the right middle lobe: pulmonary vein—junction between the oblique and horizontal fissures—pulmonary artery—poorly developed fissure—bronchus; (III) the right lower lobe: pulmonary vein—oblique fissure—pulmonary artery—poorly developed fissure—bronchus; (IV) the left upper lobe: pulmonary vein—pulmonary artery—oblique fissure—posterior ascending branch of the pulmonary artery—poorly developed fissure—bronchus; (V) the left lower lobe: pulmonary vein—oblique fissure—pulmonary artery—poorly developed fissure—bronchus. In the unidirectionally progressive lobectomy, the operation proceeds from the soft tissue at the hilum to deeper structures through the working port, in which the layers are freed and separated successively until the fissure in a single direction. This eliminates the need for flipping the lobes back and forth, up and down. The resection is completed unidirectionally from the anterior to posterior regions when removing the upper or middle lobes, or from the inferior to superior regions when dealing with the lower lobe. The pulmonary vein, bronchi, pulmonary artery and the poorly developed fissure of the right lower lobe are treated successively during lobectomy. In view of the specific structure of the pulmonary hilum, the operation is progressed in the order of freeing and separating the pulmonary vein, the bronchus, the pulmonary artery, and the pulmonary fissure. In the case of resection of the right upper lobe, the superior pulmonary vein is initially freed. A stapler is inserted through the auxiliary port for dissection of the right superior pulmonary vein, and separation of all branches of the superior pulmonary artery, which are cut with the stapler or ligated with suture. The upper lobe bronchus is then freed towards the posterior region, and cut with the stapler through the auxiliary port. At last, based on the development conditions, the fissure is freed with an electrome or the stapler. The hilar structures are mainly freed with the electrome in combination with a suction device. In this video, unidirectionally progressive left pneumonectomy is performed in the order of left superior pulmonary vein—left lower pulmonary vein—left main bronchus—left pulmonary artery. The vessels and bronchi are cut using an endoscopic linear stapler or the Hemolock clips (Figures 5-14).
Mediastinal lymph node dissection is then conducted, and the surgery ends.
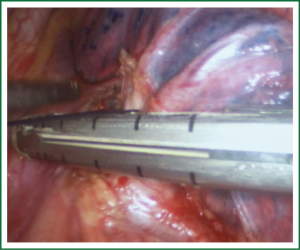
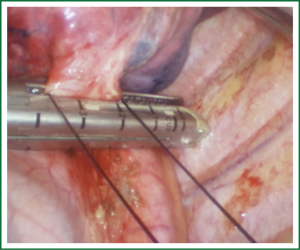
Comments
Using complete thoracoscopic lung resection, the operators can have a thorough understanding of the tissue in the chest cavity through the video monitor. During the operation, the operators can observe any area inside the cavity, and observe and operate through the thoracoscope, without any blind spot. The high brightness provided by thoracoscopy ensures clearer surgical field and thus more stable operation. The local magnification enables safe and fine surgery, avoiding blind separation of tissue, thereby ensuring better treatment of the hilar vessels and main bronchi compared with open thoracotomy (6). With complete thoracoscopic lung resection, the incision can be as small as only 3 to 5 cm, thus avoiding the use of thoracotomy and extensive cutting of the chest muscles, allowing better protection of the neuromuscular system and significantly reducing postoperative pain, which is the most common complication after chest surgery and a major compromising factor to postoperative recovery (7). Therefore, minimally invasive surgery is more conducive to postoperative rehabilitation, and more easily acceptable for patients, empowering them with better confidence in recovery. Since the patient is treated with VATS lobectomy, the key to success is similar to traditional lobectomy, which includes thorough lymph node dissection, and proper management of major intraoperative bleeding. The lobectomy should be followed by systemic lymph node dissection. The dead spot-free coverage and certain amplification of the surgical field in VATS allow further thorough lymph node dissection compared with traditional open surgery. However, obviously enlarged lymph nodes often indicate the presence of metastases, which are associated with a high risk of capsule rupture and potential tumor seeding during thoracoscopic resection. Therefore, we do not recommend this surgical technique for patients with known significant enlargement of hilar or mediastinal lymph nodes, or as confirmed by preoperative PET-CT or CT. At present, however, complete thoracoscopic surgery is only available in large hospitals in China, mainly due to the strict demands for highly experienced surgeons. The major difficulty in managing accidental bleeding from pulmonary blood vessels is also a primary cause of conversion to thoracotomy. Enlarged lymph nodes caused by inflammation, tuberculosis and tumor metastasis often obscure the local anatomy, and increase the difficulty of endoscopic treatment of vascular and bronchial structures. In such case, a slightest carelessness may result in injury to the blood vessels and the consequent bleeding and increased risk of conversion to open surgery (8). When intraoperative vascular hemorrhage occurs, the operator must stay calm and do not panic. Using forceps to repeatedly clamp the bleeding vessels is strictly forbidden. Otherwise, more bleeding is likely to be the result. At this point, the big “peanuts”—prepared multi-folded gauze—on the operating table should be used for hemostasis. Alternatively, a large toothless oval clamp can be directly applied to clamp the bleeding site for temporary control, followed by timely conversion to thoracotomy to repair the blood vessels in order to ensure safety. The unidirectional procedure of VATS lobectomy has shaken off the shackles of traditional process, making it easier to perform the lobectomy under thoracoscope in a smooth and simple way. It also reduces lung injury by avoiding repeated flipping of the lung tissue.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- He JX. Atlas of minimally invasive thoracic surgery. Guangzhou: Guangdong Science and Technology Publishing House, 2005:195-228.
- Pu JT, Dai TY, Tang XJ, et al. VATS Thoracic Small Incision and Conventional Surgery for Lung Cancer: Comparison of Therapy Effect and Impact on Blood Coagulation. Chinese Modern Doctor 2010;48:5-7.
- Seder CW, Hanna K, Lucia V, et al. The safe transition from open to thoracoscopic lobectomy: a 5-year experience. Ann Thorac Surg 2009;88:216-25; discussion 225-6. [PubMed]
- Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22. [PubMed]
- Liu XL, Che GW, Pu Q, et al. Single-direction VATS lobectomy. Chinese Journal of Thoracic and Cardiovascular Surgery 2008;24:156-8.
- Zhou HY, Ye X, Chen G, et al. Primary outcome of completely thoracoscopic pneumonectomy for non-smaU cell lung cancer. Chinese Journal of Thoracic and Cardiovascular Surgery 2013;29:1-3.
- Khoshbin E, Al-Jilaihawi AN, Scott NB, et al. An audit of pain control pathways following video-assisted thoracoscopic surgery. Innovations (Phila) 2011;6:248-52. [PubMed]
- Sugi K, Sudoh M, Hirazawa K, et al. Intrathoracic bleeding during video-assisted thoracoscopic lobectomy and segmentectomy. Kyobu Geka 2003;56:928-31. [PubMed]