High blood neutrophil-lymphocyte ratio associated with poor outcomes in miliary tuberculosis
Introduction
Miliary tuberculosis (TB) is a potentially life-threatening disease caused by lymphohematogenous dissemination of Mycobacterium tuberculosis (1,2). Since a clinical course of miliary TB is nonspecific and quite variable, it is difficult to predict a prognosis early in the disease. Manifestations are likely to be subacute or chronic, but can be rapidly developed into fulminant state such as acute respiratory distress syndrome (ARDS) or septic shock with multi-organ failure (3-7).
Mortality rate of miliary TB has been reported to be 25–30% and up to 65% for patients with mechanical ventilation (8-10). Predictors for miliary TB in previous studies have been found to be aging, female sex, presence of underlying comorbidity, hyponatremia, hypoalbuminemia, leukopenia, elevated transaminase level, and poor nutritional status (2,6,9-15). However, the aforementioned factors have not demonstrated consistent results.
Neutrophil-lymphocyte ratio (NLR) is defined as the number of neutrophils in whole blood divided by the number of lymphocytes in whole blood (16). It draws attention as recent demonstration of a readily available laboratory marker used to estimates systemic inflammation has been found to be a useful biomarker for predicting mortality in various clinical setting including cancers and coronary heart diseases (17-21). In case of respiratory diseases, it has been studied mainly for lung cancer (22,23). Although NLR has been shown to be useful for differentiating TB from bacterial pneumonia or sarcoidosis in a few studies on TB (24,25), the association of NLR with clinical outcome in patients with miliary TB has not been reported. We hypothesized that NLR could reflect independent prognostic significance in patients with miliary TB.
Methods
Study design and data collection
We conducted a retrospective cohort study on hospitalized patients diagnosed with miliary TB at the Ewha Womans University Mokdong Hospital in Korea from January 1996 to May 2015. Following acquisition of disease code for miliary TB based on code designation by the National Health Service Center of Korea, we searched for patients on monitoring track with such code for miliary TB. Among these patients, we excluded those who were already diagnosed with miliary TB and on TB medication prior to being hospitalized (Figure 1).
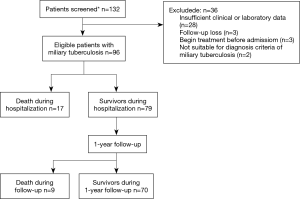
We defined the diagnosis of miliary TB with appearance of lung nodule with miliary pattern reported by a board-certified radiologist as a result of chest radiography and computed tomography. In addition, assessment of TB was carried out to find a match with at least one of the following criteria: (I) positive smear for acid-fast bacilli or positive polymerase chain reaction or culture for M. tuberculosis in specimens of sputum, bronchoalveolar lavage fluid, pleural fluid or other tissue specimen; (II) histopathologic implication of TB infection in tissue specimen such as lung biopsy or lymph node biopsy; and (III) radiological improvement after anti-TB treatment.
We reviewed the medical records and examined age, sex, height, weight, symptoms, comorbidity, medication history, duration of hospitalization, duration of intensive care unit (ICU) admission, and development of ARDS. In order to identify factors relevant to mortality, laboratory data were collected based on the first available data at the time of admission and included complete blood cell counts (CBC), chemistry panel, inflammatory biomarkers such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The definition of clinical outcome is as follows; ARDS was defined as a clinical situation satisfying the Berlin criteria (26). In-hospital mortality was defined as death in hospital, and 1-year mortality was defined as death within 1 year after diagnosis of miliary TB. Mortality included all deaths due to other causes as well as death from miliary TB.
Our study protocol was approved by the institutional review board of the Ewha Womans University Mokdong Hospital (IRB number: 12-09A-36).
Statistical analysis
Descriptive and frequency analyses of the data were presented in mean with standard deviation and numbers with percentage, respectively. Unpaired T-test and Chi-square test or exact test was conducted where applicable. Logistic regression and Cox regression were performed for univariate and multivariate analysis to evaluate the predictors for prognosis for patients with miliary TB. The area under the curve (AUC) was calculated from the receiver operating characteristic (ROC) curve to compare the predictability of several inflammatory markers. Kaplan-Meier survival curves of two groups were compared using the log rank test. P-values less than 0.05 were considered to denote statistical significance. All data were analyzed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA).
Results
Among a total of 96 patients diagnosed with miliary TB, in-hospital mortality was 18%, and 1-year mortality was 27% (Figure 1).
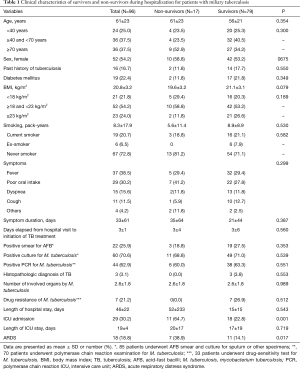
Table 1 shows comparison of clinical characteristics between survivors and non-survivors. There was no statistically significant difference in gender and age distribution between survivors and non-survivors. The most common symptoms for miliary TB included fever, poor oral intake, and dyspnea. Twenty-nine patients (30%) were admitted to the ICU, and the number of patients accompanied with ARDS was 18 (19%) and more common in the non-survivors (P=0.017). Of the 33 patients who underwent drug susceptibility testing for M. tuberculosis, seven patients were drug resistant and belonged to the survivor group. In the non-survivor group, however, only seven patients were tested for drug susceptibility and all were drug-sensitive. Of the seven drug-resistant patients, two were diagnosed with multidrug-resistant TB and two with extended multidrug-resistant TB. Three patients were resistant to rifampin alone.
Full table
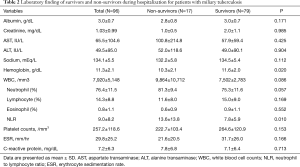
Compared with survivors, hemoglobin level was lower (P=0.02) and NLR was higher in the non-survivors (P=0.01). There was no significant statistical difference in the other laboratory tests (Table 2).
Full table
In univariate analysis, difference of clinical characteristics between patients with ARDS and those without ARDS was not found. However, in the baseline laboratory tests, the levels of serum albumin, sodium, and platelet counts were lower and the levels of CRP, ESR, and NLR were higher in patients with ARDS than those without ARDS. Except for ESR, there was a correlation between these laboratory variables. Therefore, multiple logistic regression models for ARDS including age, sex, NLR, and ESR were obtained. Only NLR remained as a significant variable for the development of ARDS (Table 3).

Full table
For in-hospital mortality, hemoglobin, aspartate aminotransferase (AST), NLR were identified as significant variables from univariate analysis. These variables remain independently valid in Cox proportional hazard model with adjustment of sex and age. For one-year mortality, age, sex, hemoglobin, AST, BMI and NLR appeared to be significant in univariate analysis. Cox proportional hazard model showed that old age, male, low hemoglobin, high AST, and high NLR were associated to 1-year mortality (Table 4).

Full table
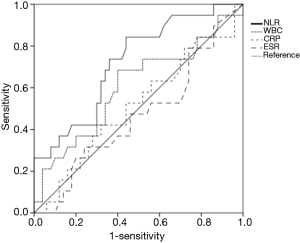
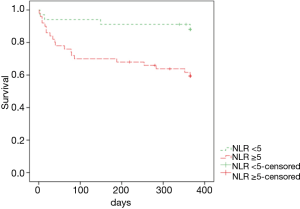
When areas under the ROC curves were analyzed to evaluate usefulness of several inflammatory markers in predicting 1-year mortality of patients with miliary TB, the AUC of the NLR (0.722) was the largest (Figure 2). Using the ROC curve analysis, the cut-off value of NLR for one-year mortality was set at 5.2, and remarkable decrease in survival rate was observed in the group with NLR ≥5 in the Kaplan-Meier curves at follow-up (log rank test, P=0.005) (Figure 3).
Discussion
Our study demonstrated that NLR is the most powerful predictor for both mortality and the occurrence of ARDS in patients with miliary TB. NLR indicates relative neutrophil elevation and lymphocyte decrease and is an inflammatory marker that can be easily obtained at low cost. For prediction of 1-year mortality in miliary TB, NLR was more significant than other inflammatory markers such as WBC, CRP and ESR based on the AUC using ROC. Association of high NLR with high mortality in cancers or cardiovascular diseases has been reported in a number of studies (18,20,23,27,28). In patients with lung cancer, high NLR may have a higher cancer stage in the perioperative period (27) and predict poor prognosis after chemotherapy (22). Explanation for the correlation between the increase in NLR and poor outcome has not yet been clarified. Although lymphopenia has been reported to be associated with poor prognosis in several previous analyses on TB (1,10,29), NLR has not been investigated in miliary TB. In cancer patients, some studies hypothesized that relatively low lymphocyte counts are associated with a poor response to chemotherapy in relation to cell-mediated immunity (22,30). Cell-mediated immunity is important in the immune response to M. tuberculosis and high NLR may reflect relative lymphopenia. Therefore, the similar pathway may have affected the progression and prognosis of miliary TB. Recently, it has been reported that NLR correlate with the severity of pulmonary TB (31) and high NLR seems to increase the likelihood of retreatment of pulmonary TB (32).
Progression of miliary TB to ARDS may occur as part of a multi-organ dysfunction syndrome or in association with immune reconstitution inflammatory syndrome (IRIS) (6,8,14,33,34). Coexistence of miliary TB and ARDS links to high mortality, which is already shown in previous studies on miliary TB (6,7). A multicenter study in Korea reported that mortality rate of ARDS patients with miliary TB was 61.2%, which was higher than mortality in ARDS patients with other causes (7). There has not been much data on the prognosis of miliary TB patients with ARDS, thus it is difficult to provide an exact explanation. Inferring the reason for this, it can be considered that the diagnosis of miliary TB is likely to be delayed and nosocomial pneumonia is frequently accompanied (35). It is not easy to suspect and diagnose miliary TB because of ARDS, and the guideline of effective treatment for ARDS is not yet established (6,7). In our study, there were 18 miliary TB patients with ARDS, which was 19% of the entire patients enrolled, and among them, 39% (7/18) died during hospitalization. This figure was lower than other studies (6,7), but 1-year mortality was similar as it being 55%. This gap of in-hospital mortality rate might be due to differences in concomitant disease or initial severity.
Several nutritional factors are also known to be involved in poor prognosis in TB including miliary TB (1,10,11,29). Our study found no significant difference in mortality associated with BMI. However, mean albumin and sodium levels were lower in non-survivors compared to those in survivors, which may be linked to nutritional status or secretory abnormalities of antidiuretic hormone (29,36).
We demonstrated that NLR is helpful in predicting poor prognosis in miliary TB patients; however, our research has some limitations. Not only the study was performed in retrospective setting but also long study period was required to enroll a large number of patients with miliary TB. Although our study is an institutional study, it is possible that the patient’s treatment was inconsistent care because of the long study period. Nevertheless, this study provides clinical value in that it offers a relatively large number of patients with rare diseases such as miliary TB and presents NLR as a new biomarker to predict prognosis.
Conclusions
Our study demonstrated that elevated NLR is associated with an increased risk of poor outcome such as in-hospital and 1-year mortality and the development of ARDS in patients with miliary TB. If patients with miliary TB have an NLR greater than 5, prompt investigation and active treatment is needed.
Acknowledgements
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2010-0027945). We thank Dr. Hye Sung Park for collecting some of the data.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our study protocol was approved by the institutional review board of the Ewha Womans University Mokdong Hospital (IRB number: 12-09A-36).
References
- Sharma SK, Mohan A, Sharma A, et al. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis 2005;5:415-30. [Crossref] [PubMed]
- Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res 2012;135:703. [PubMed]
- Piqueras AR, Marruecos L, Artigas A, et al. Miliary tuberculosis and adult respiratory distress syndrome. Intensive Care Med 1987;13:175-82. [Crossref] [PubMed]
- Sydow M, Schauer A, Crozier T, et al. Multiple organ failure in generalized disseminated tuberculosis. Respir Med 1992;86:517-9. [Crossref] [PubMed]
- Ahuja SS, Ahuja SK, Phelps KR, et al. Hemodynamic confirmation of septic shock in disseminated tuberculosis. Crit Care Med 1992;20:901-3. [Crossref] [PubMed]
- Kim JY, Park Y, Kim Y, et al. Miliary tuberculosis and acute respiratory distress syndrome. Int J Tuberc Lung Dis 2003;7:359-64. [PubMed]
- Lee K, Kim J, Lee J, et al. Acute respiratory distress syndrome caused by miliary tuberculosis: a multicentre survey in South Korea. Int J Tuberc Lung Dis 2011;15:1099-103. [Crossref] [PubMed]
- Penner C, Roberts D, Kunimoto D, et al. Tuberculosis as a primary cause of respiratory failure requiring mechanical ventilation Am J Respir Crit Care Med 1995;151:867-72. [Crossref] [PubMed]
- Al-Jahdali H, Al-Zahrani K, Amene P, et al. Clinical aspects of miliary tuberculosis in Saudi adults. Int J Tuberc Lung Dis 2000;4:252-5. [PubMed]
- Kim DK, Kim H, Kwon S, et al. Nutritional deficit as a negative prognostic factor in patients with miliary tuberculosis. Eur Respir J 2008;32:1031-6. [Crossref] [PubMed]
- Hussain SF, Irfan M, Abbasi M, et al. Clinical characteristics of 110 miliary tuberculosis patients from a low HIV prevalence country. Int J Tuberc Lung Dis 2004;8:493-9. [PubMed]
- Long R, O'Connor R, Palayew M, et al. Disseminated tuberculosis with and without a miliary pattern on chest radiograph: a clinical–pathologic–radiologic correlation. Int J Tuberc Lung Dis 1997;1:52-8. [PubMed]
- Maartens G, Willcox PA, Benatar SR. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med 1990;89:291-6. [Crossref] [PubMed]
- Mohan A, Sharma S, Pande J. Acute respiratory distress syndrome (ARDS) in miliary tuberculosis: a twelve year experience. Indian J Chest Dis Allied sci 1996;38:157-62. [PubMed]
- Underwood J, Cresswell F, Salam AP, et al. Complications of miliary tuberculosis: low mortality and predictive biomarkers from a UK cohort. BMC Infect Dis 2017;17:295. [Crossref] [PubMed]
- Imtiaz F, Shafique K, Mirza SS, et al. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med 2012;5:2. [Crossref] [PubMed]
- Wyllie DH, Bowler I, Peto T. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol 2004;57:950-5. [Crossref] [PubMed]
- Papa A, Emdin M, Passino C, et al. Predictive value of elevated neutrophil–lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta 2008;395:27-31. [Crossref] [PubMed]
- Walsh SR, Cook E, Goulder F, et al. Neutrophil‐lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181-4. [Crossref] [PubMed]
- Núñez J, Núñez E, Bodí V, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol 2008;101:747-52. [Crossref] [PubMed]
- Chua W, Charles K, Baracos V, et al. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 2011;104:1288-95. [Crossref] [PubMed]
- Yao Y, Yuan D, Liu H, et al. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother 2013;62:471-9. [Crossref] [PubMed]
- Cedrés S, Torrejon D, Martinez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol 2012;14:864-9. [Crossref] [PubMed]
- Yoon N-B, Son C, Um S-J. Role of the neutrophil-lymphocyte count ratio in the differential diagnosis between pulmonary tuberculosis and bacterial community-acquired pneumonia. Ann Lab Med 2013;33:105-10. [Crossref] [PubMed]
- Iliaz S, Iliaz R, Ortakoylu G, et al. Value of neutrophil/lymphocyte ratio in the differential diagnosis of sarcoidosis and tuberculosis. Ann Thorac Med 2014;9:232. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425-8. [Crossref] [PubMed]
- Okamura K, Nagata N, Wakamatsu K, et al. Hypoalbuminemia and lymphocytopenia are predictive risk factors for in-hospital mortality in patients with tuberculosis. Intern Med 2013;52:439-44. [Crossref] [PubMed]
- Sutherland JS, Jeffries DJ, Donkor S, et al. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis 2009;89:398-404. [Crossref] [PubMed]
- Abakay O, Abakay A, Sen HS, et al. The relationship between inflammatory marker levels and pulmonary tuberculosis severity. Inflammation 2015;38:691-6. [Crossref] [PubMed]
- Yin Y, Kuai S, Liu J, et al. Pretreatment neutrophil-to-lymphocyte ratio in peripheral blood was associated with pulmonary tuberculosis retreatment. Arch Med Sci 2017;13:404-11. [Crossref] [PubMed]
- Sharma SK, Mohan A, Banga A, et al. Predictors of development and outcome in patients with acute respiratory distress syndrome due to tuberculosis. Int J Tuberc Lung Dis 2006;10:429-35. [PubMed]
- Goldsack NR, Allen S, Lipman M. Adult respiratory distress syndrome as a severe immune reconstitution disease following the commencement of highly active antiretroviral therapy. Sex Transm Infect 2003;79:337-8. [Crossref] [PubMed]
- Erbes R, Oettel K, Raffenberg M, et al. Characteristics and outcome of patients with active pulmonary tuberculosis requiring intensive care. Eur Respir J 2006;27:1223-8. [Crossref] [PubMed]
- Sharma SK, Mohan A, Pande J, et al. Clinical profile, laboratory characteristics and outcome in miliary tuberculosis. QJM 1995;88:29-37. [PubMed]


