Physiological consequences of CPAP therapy withdrawal in patients with obstructive sleep apnoea—an opportunity for an efficient experimental model
Introduction
Continuous positive airway pressure (CPAP) is the gold standard treatment for obstructive sleep apnoea (OSA) (1-4). CPAP is very effective in abolishing apnoea and hypopnoea, and reverses the pathophysiologic consequences of OSA. Its effectiveness depends on sufficient and regular usage.
Randomised controlled trials (RCTs) in patients with moderate-to-severe OSA have shown that treatment with CPAP reduces excessive daytime sleepiness (1) and improves driving performance (5) and health related-quality of life (1). However, many conclusions as to the effects of CPAP treatment in OSA come from population-based epidemiological studies, and robust evidence from randomised controlled interventional trials is often missing. There is still a need for well-designed RCTs to study the effects of CPAP therapy on diverse physiological and clinically relevant outcomes, e.g., cardio- and cerebrovascular events. Conventional CPAP trials are typically limited by the unpredictable and often suboptimal CPAP adherence in treatment-naïve patients, which lead to underestimation of treatment effects. Subjects who do not tolerate CPAP well, and use it only intermittently during the night, might even have negative effects such as disturbed sleep architecture and elevated blood pressure (6). This suboptimal CPAP adherence hampers the interpretation of the often cumbersome and expensive conventional CPAP RCTs. Furthermore, there is interest in novel treatment modalities as alternatives to CPAP, since the latter is not tolerated by all patients, and compliance with this treatment is often suboptimal. Thus, a rapid assessment tool for these new treatments would be helpful. We therefore suggest a short-term CPAP withdrawal as an effective study model to investigate both the consequences of untreated OSA, and potential treatment alternatives to CPAP. What evidence exists to justify this approach?
The CPAP withdrawal model
In the CPAP withdrawal model, patients previously diagnosed with OSA, and effectively treated and compliant with CPAP, are randomised to either continue therapeutic CPAP or to withdraw it by using a subtherapeutic sham-CPAP device, for a short period (e.g., 2 weeks). This controlled model can be conducted with double-blind randomisation. Measurements are performed at baseline on CPAP, and at follow-up on either CPAP (control group) or sham-CPAP (intervention group) (see Figure 1). Additional repeated measurements after randomisation over the experimental period can be inserted to assess the gradual changes in the outcomes. Furthermore, the traditional two-arm CPAP withdrawal model can be extended to a several-arm RCT to study therapy alternatives by adding one or more extra CPAP-withdrawal arms using the novel treatment(s). Therefore, the novel treatments can be compared to both the continuing on CPAP, and/or to the withdrawal group serving as an untreated control group with full return of OSA.
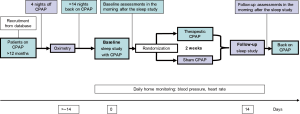
This model can study medically well-characterised and carefully selected patients with OSA from large cohorts who are already being treated with CPAP. Sleep centres or CPAP providers usually have databases of treated OSA patients, including information on their therapy compliance, thus allowing fast recruitment. Carefully selected eligibility criteria (e.g., inclusion of patients with high long-term CPAP adherence and exclusion of professional drivers) can be utilised to make the design effective and safe.
In general, persistence of OSA is first confirmed before study inclusion by home overnight pulse-oximetry at the end of a four-night period off CPAP. This is important since the time of diagnosis of OSA and CPAP initiation might have been some years ago, and potential causal factors, such as obesity, might have changed.
Discussion
Physiological effects of CPAP withdrawal
We have shown that a short-term CPAP withdrawal results in recurrence of OSA as indicated by changes in sleep study parameters. This recurrence of OSA in response to CPAP therapy withdrawal was associated with a number of consequences: a deterioration of daytime symptoms and psychomotor performance; increases in blood pressure, heart rate, and urinary catecholamines; peripheral endothelial dysfunction; disturbances of cardiac repolarisation; and changes in the metabolic breath profile (7-10). However, we have also demonstrated that short-term CPAP withdrawal does not result in impaired myocardial perfusion, despite the increase in blood pressure and other adverse effects of OSA (11). This was an important message for patients with OSA, and for physicians treating such patients, since taking a CPAP holiday, for several days or more, is common even in compliant patients. Contrary to our prior hypothesis, we found that the return of intermittent hypoxia did not increase oxidative stress, but may have decreased it, potentially explained by hypoxic preconditioning (12). The most important findings of the trials using the CPAP withdrawal protocol are described below (also see Figures 2,3).

The first proof of concept study defined the physiological effects of CPAP withdrawal on OSA recurrence, daytime symptoms, blood pressure, endothelial function, and systemic inflammation at 1 and 2 weeks after CPAP withdrawal (7). Withdrawal of CPAP resulted in an increased apnoea-hypopnoea-index (AHI) at 1 and 2 weeks to a comparable degree (mean increase in AHI +31.9 (95% CI: +20.1 to +43.7) and +33.5 (95% CI: +22.4 to +44.6), respectively; P<0.001 for both comparisons) compared to continuation of CPAP (7). However, subjective sleepiness, as assessed by the Epworth Sleepiness Scale (ESS), increased gradually at 1 and 2 weeks in the CPAP withdrawal group, compared to the control group (mean rise in ESS +1.9 (95% CI: +0.4 to +3.3) and +2.7 (95% CI: +1.2 to +4.3); P=0.015 and P<0.001, respectively) (7). Despite increased daytime sleepiness, short-term CPAP withdrawal was not associated with deterioration in psychomotor performance (divided attention driving simulator and psychomotor vigilance task) (7). This study established the CPAP withdrawal model and has shown that CPAP therapy withdrawal usually leads to a rapid recurrence of OSA, accompanied by a gradual return of subjective daytime sleepiness. Additionally, this RCT showed that therapy withdrawal in OSA leads to increases in markers of sympathetic activity (but not systemic inflammation) and endothelial dysfunction as assessed by flow-mediated dilatation of the brachial artery —a well validated method to assess endothelial function (7).
Based on these findings, the hypothesis was postulated that CPAP withdrawal also results in dysfunction of the microvasculature, and therefore in impairment of myocardial perfusion. To answer this question, a randomised controlled CPAP withdrawal trial assessing the change in myocardial blood flow during adenosine-induced vasomotor stress assessed by 13N-ammonia positron emission tomography was conducted (11). This is the current gold standard for the quantification of myocardial blood flow (13). We found that myocardial blood flow does not change in response to recurrence of moderate to severe OSA after 2 weeks of CPAP withdrawal when compared to continuing CPAP therapy (11). CPAP withdrawal also had no effect on other microvascular beds, e.g., the dermal microcirculation (11). The confidence intervals of the treatment effect clearly demonstrated that any relevant effect on myocardial perfusion can be excluded (treatment effect on hyperaemic myocardial blood flow: −0.01 mL/min/g, 95% CI: −0.33 to +0.24 mL/min/g, P=0.91; minimally clinically important difference 0.75 mL/min/g) (11). This finding was unexpected because, in theory, recurrence of OSA may impair myocardial perfusion by several possible mechanisms, including endothelial dysfunction that could result from augmented sympathetic activity, increased oxidative stress due to intermittent hypoxia, as well as increased oxygen demand from the increased cardiac work load due to accelerated heart rate and elevated blood pressure. However, this study clearly showed that there is no immediate adverse effect of CPAP therapy withdrawal on myocardial perfusion in patients with moderate to severe OSA, despite the recurrence of OSA and relevant increases in blood pressure. We concluded that OSA patients are unlikely to be at risk for acute myocardial ischemia during short periods of treatment interruption such as on vacation. This finding was an important message for both clinicians and patients with OSA, since “CPAP holidays” are common.
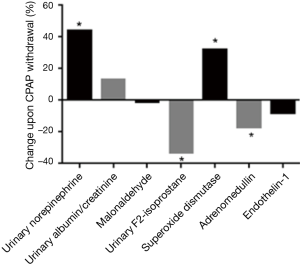
Whereas there is robust evidence for increased sympathetic activity as the most relevant contributing factor for the systemic consequences of OSA, there is inconsistent data on the role of intermittent hypoxia, oxidative stress and systemic inflammation as potential cause of cardiovascular consequences. In order to examine whether withdrawal of CPAP and thus reactivation of intermittent hypoxia would result in a rise of markers of oxidative stress, 59 patients with moderate to severe OSA were randomised to either continue therapeutic CPAP or to subtherapeutic CPAP (sham) (12). However, despite re-occurrence of intermittent hypoxia, there was no evidence of an increase in markers of oxidative stress in response to recurrence of OSA, e.g., early morning blood malondialdehyde—a sensitive marker of oxidative stress. Unexpectedly, OSA reactivation was associated with a significant reduction in urinary F2-isoprostane (P=0.002 compared to the control group) that showed a correlation with the oxygen desaturation index (ODI) (r=−0.41, P=0.001) (12). However, this finding was confounded by a rise in overnight urinary volume in those with returning OSA (14). This finding implied a reduction in oxidative stress and possible explanation was a significant increase in superoxide dismutase—a marker of hypoxic pre-conditioning—found in patients with returning OSA compared to those remaining on CPAP (12).
Exhaled breath contains metabolic information on the pathophysiological state. We used the CPAP withdrawal protocol to test whether a disease specific profile of exhaled breath in patients with OSA can be detected by real-time exhaled breath analysis by mass spectrometry. Untargeted exhaled breath analysis was used to define the effect of metabolic changes on the breath profile (10).
Indeed, recurrence of OSA was accompanied by a specific change in exhaled breath pattern. The panel of discriminating mass-spectral features allowed separation between treated and untreated OSA with a sensitivity of 92.9% and a specificity of 84.6% (10). There was also a good correlation between changes in the breath signal intensity of these features, and the change in ODI as a measure of OSA severity (10). These data on OSA-specific compounds in exhaled breath supported the association of OSA with increased sympathetic activity and lipid peroxidation, as well as evidence of a change in gut flora. This withdrawal study has shown that exhaled breath analysis by real-time untargeted mass spectrometry allows rapid and non-invasive recognition of returning OSA with good sensitivity and specificity, as well as the identification of new biomarkers. This approach of breath profiling in OSA might be useful in both screening for OSA as well as monitoring treatment adherence.
Using data from this experimental protocol, we have tested additional hypotheses based on preliminary evidence from other experimental studies, with a focus on mechanisms explaining the observed increased incidence of cardiovascular disease in OSA. The blood pressure lowering effect of CPAP therapy, in meta-analyses based on RCTs in previously untreated patients, is probably underestimated due to suboptimal CPAP usage (15). To measure the withdrawal effect on blood pressure and to find potential clinical predictors of blood pressure response to OSA treatment, data from 149 optimally CPAP compliant patients, included in randomised-controlled CPAP withdrawal trials, were analysed (8). Consistent with the previous smaller withdrawal studies, there was a significant increase in morning blood pressure and heart rate in the withdrawal group compared to the continuing therapeutic CPAP group. OSA recurrence was associated with a clinically relevant increase in home systolic and diastolic blood pressure of approximately 9 and 8 mmHg, respectively (8). This effect on blood pressure is considerably higher than in conventional CPAP trials. This might be explained by a maximal treatment or withdrawal effect due to the high prior CPAP adherence in the study population. The effect on blood pressure was underestimated when office values, instead of home values, were used. With this in mind, home blood pressure, instead of office blood pressure measurements, should probably be used as the most sensitive blood pressure outcome in clinical trials.
This analysis has established that the blood pressure lowering effects of CPAP are highest in those with the most severe OSA, and in those on several antihypertensive drugs (8).
In addition to systemic hypertension, blood pressure variability has been suggested as an independent cardiovascular risk factor (16). However, within the CPAP withdrawal trials, randomising 183 patients to either continue therapeutic CPAP or to withdraw it, there was only a negligible increase in within-visit variability in systolic office blood pressure, whereas there was no effect of withdrawing CPAP on other short-term blood pressure variability indices (within-visit), or on intermediate-term blood pressure variability indices (visit-to-visit, day-to-day) (17). The hypothesis that OSA contributes to vascular damage via elevated daytime blood pressure variability could not be supported.
Untreated OSA has been associated with cardiac arrhythmias and increased incidence of sudden cardiac death (18,19). Disturbed cardiac repolarisation, induced by autonomic dysfunction, has been suggested as a potential underlying mechanism of arrhythmogenesis. Analysing electrocardiographic data from a CPAP withdrawal study, we found that CPAP withdrawal led to a statistically significant prolongation of repolarisation and an increase in the dispersion of transmural cardiac repolarisation compared to continuing therapeutic CPAP (9). These changes showed a good correlation with the change in AHI (9). These findings provided a potential mechanistic link between OSA and cardiac arrhythmias.
Patients with OSA suffer from cognitive impairment and have an increased risk of stroke in observational studies (20). Using the randomised controlled CPAP withdrawal protocol, it was demonstrated that OSA is associated with intermittent and sustained nocturnal cerebral tissue hypoxia, to a clinically relevant degree reported to cause functional impairment (21,22). This study suggested that CPAP may prevent the risk of nocturnal ischaemic cerebral damage.
Most data on the association between OSA and possible activation of (hypoxia-induced) inflammatory pathways, potentially promoting the observed adverse cardiovascular risk, comes mainly from cell culture, animal or case control studies. RCTs on the effect of CPAP therapy on markers of systemic inflammation are inconclusive (23,24). Interpretation is additionally complicated due to the common co-existence of OSA and obesity (potential adipose tissue-mediated inflammation). In 109 patients with OSA included in CPAP withdrawal trials (a real life model of intermittent hypoxia) levels of selected vascular inflammatory markers linked to hypoxia and previously reported to be altered in OSA were analysed; the endothelium-derived protein endocan, the endothelial-derived vasodilator adrenomedullin, and the vasoconstrictor endothelin-1 (25). Of interest, CPAP withdrawal led to a small but significant decrease in adrenomedullin compared to continuation of therapeutic CPAP. However levels of hypoxia-induced and other endothelial-derived inflammatory markers and vasoconstrictive substances were unchanged (25). This is not in line with observational studies in OSA and non-human models of intermittent or sustained hypoxia. The return of OSA upon CPAP withdrawal was associated with a significant increase in endothelial cell-derived microvesicles (26). Of interest, a more specific exploratory analysis revealed a significant increase in platelet-derived and procoagulant microvesicles in response to CPAP withdrawal (27). However, the hypothesis of potential effects of OSA on the coagulation system has to be further tested.
Currently, there are ongoing randomised controlled CPAP withdrawal trials to establish the effects of OSA on retinal vascular reactivity (ISRCTN78082983), and on cerebrovascular reactivity (NCT02493673). Additionally, there is an ongoing CPAP withdrawal trial to evaluate whether patients with suboptimal CPAP usage still benefit from treatment or not (NCT02781740).
The withdrawal model for assessment of treatment alternatives
The CPAP withdrawal model can not only be applied to evaluate the pathophysiological consequences of OSA, but also to rapidly study the response to novel treatment approaches. The Provent® trial was a three-arm RCT studying the efficacy of a nasal expiratory positive airway pressure device to prevent the recurrence of OSA following the CPAP withdrawal. Sixty-seven patients with OSA, previously on CPAP, were randomised to either continue CPAP or to use the nasal EPAP-device or a sham-nasal EPAP-device (28). The trial found no therapeutic effect of Provent® on OSA compared to sham-Provent, and thus concluded that the nasal EPAP device is ineffective and cannot be recommended as an alternative treatment for OSA. Recently, a CPAP withdrawal RCT on the effects of supplemental oxygen versus sham oxygen on the blood pressure rise seen following the return of OSA (ISRCTN17987510) was completed. This trial hopes to answer the question whether intermittent hypoxia is, or is not, the major cause of the rise in diurnal blood pressure in OSA (29), as supplemental oxygen greatly attenuates the hypoxic dips without significantly affecting the AHI or arousals (30). Preliminary results suggest that the blood pressure rise following CPAP withdrawal is significantly attenuated by the supplemental oxygen (30).
Other studies using therapy withdrawal in OSA
The concept of studying the pathophysiological consequences of OSA by a CPAP therapy withdrawal has been also been applied by other groups (31). However, a short-term withdrawal (often just for one night) has mainly been used to study OSA in uncontrolled and non-randomised studies (31-43). A return of OSA, however to a lesser extent, was described even after one night of CPAP withdrawal associated with somnolence, decreased alertness and impaired vigilance (31-43). One crossover RCT used CPAP withdrawal to study the effects of OSA on metabolic markers (44). Another RCT found an improvement in olfactory function on CPAP which was reversed upon CPAP withdrawal (45). Additionally, one group also used CPAP therapy withdrawal to study modafinil as a treatment alternative (46-48). They finally concluded that modafinil is a potential short-term treatment option during acute CPAP withdrawal since it improved performance. Withdrawal of treatment effects have not only been studied using CPAP, but also in electrical stimulation as a treatment of OSA (49).
Summary of the findings from the CPAP withdrawal model
In conclusion, the findings from the CPAP withdrawal RCTs have provided robust evidence for increased sympathetic activity in OSA associated with a considerable blood pressure increase, relevant cerebral hypoxia, and disturbed cardiac repolarisation. Furthermore, the blood pressure lowering effect of CPAP was further proven. Additionally, the findings of these trials have challenged previous hypotheses on the role of intermittent hypoxia in oxidative stress and vascular inflammation, and have suggested a so far unconfirmed mechanism of hypoxic preconditioning in OSA.
Strengths and limitations of the CPAP withdrawal model
The CPAP withdrawal model is a very effective design to study the pathophysiological consequences of OSA and treatment effects in well-characterised patients. The recruitment rate is considerably faster, since it is based on databases of cohorts of already treated patients with OSA. Additionally, the duration of the intervention is limited. These two factors make the CPAP withdrawal model very time and cost effective. An additional advantage is that the short intervention period eliminates many potential confounders that do not change in the short-term (e.g., body mass index and an obesity effect), and the model allows OSA to be studied in a controlled fashion. The major benefit over conventional intention-to-treat randomised controlled CPAP trials, using previously therapy-naïve patients, is that a CPAP withdrawal study overcomes the compliance problem by only including patients previously optimally adherent to CPAP. Thereby, a maximal treatment effect can be assumed. Treatment-naïve patients with OSA may take time to become—or never become—established on CPAP therapy during the study period. The treatment effect in conventional CPAP trials is therefore usually diluted. Additionally, the effectiveness of novel therapies on OSA and its daytime symptoms can reliably be studied and compared to both continuing CPAP, the current gold-standard, as well as untreated OSA following CPAP withdrawal.
However, a major limitation is that the consequences of OSA recurrence during a short-term withdrawal of CPAP therapy cannot be equated to any long-term effects of untreated OSA. The question of whether short- and long-term effects of untreated OSA are comparable remains unanswered. Finally, there will be a bias towards patients who are prepared to temporarily stop CPAP, which may mean that they have on average less severe OSA-related symptoms for example. Withdrawing CPAP results in increased daytime sleepiness and reduced driving performance (7,33,34). Therefore, one might raise a safety issue from withdrawing CPAP for 2 weeks. This is addressed by excluding professional drivers and patients with other vigilance-critical professions, informing patients about the expected increased sleepiness and reduced performance, and advising patients to avoid driving etc. during the study period if feeling at all sleepy. Therefore, the model is safe to evaluate treatment effects on OSA. In our experience, CPAP withdrawal has been well tolerated by the study participants, and CPAP holidays are common in real life.
Conclusions
The short-term CPAP withdrawal model offers an experimental protocol to effectively study the pathophysiological effects of OSA, as well as treatment effects in well-designed RCTs. Additionally, it can be used to provide RCT data on potential alternative treatment modalities in a head-to-head comparison with both CPAP and untreated OSA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jenkinson C, Davies RJ, Mullins R, et al. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet 1999;353:2100-5. [Crossref] [PubMed]
- Sullivan CE, Issa FG, Berthon-Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1:862-5. [Crossref] [PubMed]
- Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003;163:565-71. [Crossref] [PubMed]
- Ballester E, Badia JR, Hernandez L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med 1999;159:495-501. [Crossref] [PubMed]
- Hack M, Davies RJ, Mullins R, et al. Randomised prospective parallel trial of therapeutic versus subtherapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnoea. Thorax 2000;55:224-31. [Crossref] [PubMed]
- Bratton DJ, Stradling JR, Barbe F, et al. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax 2014;69:1128-35. [Crossref] [PubMed]
- Kohler M, Stoewhas AC, Ayers L, et al. Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2011;184:1192-9. [Crossref] [PubMed]
- Schwarz EI, Schlatzer C, Rossi VA, et al. Effect of CPAP Withdrawal on BP in OSA: Data from Three Randomized Controlled Trials. Chest 2016;150:1202-10. [Crossref] [PubMed]
- Rossi VA, Stoewhas AC, Camen G, et al. The effects of continuous positive airway pressure therapy withdrawal on cardiac repolarization: data from a randomized controlled trial. Eur Heart J 2012;33:2206-12. [Crossref] [PubMed]
- Schwarz EI, Martinez-Lozano Sinues P, Bregy L, et al. Effects of CPAP therapy withdrawal on exhaled breath pattern in obstructive sleep apnoea. Thorax 2016;71:110-7. [Crossref] [PubMed]
- Schwarz EI, Schlatzer C, Stehli J, et al. Effect of CPAP Withdrawal on myocardial perfusion in OSA: A randomized controlled trial. Respirology 2016;21:1126-33. [Crossref] [PubMed]
- Stradling JR, Schwarz EI, Schlatzer C, et al. Biomarkers of oxidative stress following continuous positive airway pressure withdrawal: data from two randomised trials. Eur Respir J 2015;46:1065-71. [Crossref] [PubMed]
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830-40. [Crossref] [PubMed]
- Turnbull CD, Akoumianakis I, Antoniades C, et al. Overnight urinary isoprostanes as a marker of oxidative stress in obstructive sleep apnoea. Eur Respir J 2017;49:1601787. [Crossref] [PubMed]
- Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA 2015;314:2280-93. [Crossref] [PubMed]
- Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010;375:895-905. [Crossref] [PubMed]
- Lettau F, Schwarz EI, Stradling JR, et al. Blood Pressure Variability in Obstructive Sleep Apnoea: Data from 4 Randomised Controlled CPAP Withdrawal Trials. Respiration 2017;93:311-8. [Crossref] [PubMed]
- Namtvedt SK, Randby A, Einvik G, et al. Cardiac arrhythmias in obstructive sleep apnea (from the Akershus Sleep Apnea Project). Am J Cardiol 2011;108:1141-6. [Crossref] [PubMed]
- Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005;352:1206-14. [Crossref] [PubMed]
- Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269-77. [Crossref] [PubMed]
- Schwarz EI, Furian M, Schlatzer C, et al. OSA results in nocturnal cerebral hypoxia which is prevented by CPAP - Data from a randomised controlled trial. Eur Respir J 2015;46:PA2333.
- Al-Rawi PG, Kirkpatrick PJ. Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke 2006;37:2720-5. [Crossref] [PubMed]
- Kohler M, Ayers L, Pepperell JC, et al. Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax 2009;64:67-73. [Crossref] [PubMed]
- Drager LF, Bortolotto LA, Figueiredo AC, et al. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 2007;176:706-12. [Crossref] [PubMed]
- Turnbull CD, Rossi VA, Santer P, et al. Effect of OSA on hypoxic and inflammatory markers during CPAP withdrawal: Further evidence from three randomized control trials. Respirology 2017;22:793-9. [Crossref] [PubMed]
- Ayers L, Stoewhas AC, Ferry B, et al. Circulating levels of cell-derived microparticles are reduced by mild hypobaric hypoxia: data from a randomised controlled trial. Eur J Appl Physiol 2014;114:1067-73. [Crossref] [PubMed]
- Ayers L, Turnbull C, Petousi N, et al. Withdrawal of Continuous Positive Airway Pressure Therapy for 2 Weeks in Obstructive Sleep Apnoea Patients Results in Increased Circulating Platelet and Leucocyte-Derived Microvesicles. Respiration 2016;91:412-3. [Crossref] [PubMed]
- Rossi VA, Winter B, Rahman NM, et al. The effects of Provent on moderate to severe obstructive sleep apnoea during continuous positive airway pressure therapy withdrawal: a randomised controlled trial. Thorax 2013;68:854-9. [Crossref] [PubMed]
- Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010;7:677-85. [Crossref] [PubMed]
- Turnbull C, Johar A, Petousi N, et al. The Effects of Supplemental Oxygen on Obstructive Sleep Apnea During CPAP Withdrawal: Preliminary Data from a Randomised Control Trial. Am J Respir Crit Care Med 2017;195:A2579.
- Phillips CL, Yang Q, Williams A, et al. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res 2007;16:217-25. [Crossref] [PubMed]
- Grunstein RR, Stewart DA, Lloyd H, et al. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep 1996;19:774-82. [Crossref] [PubMed]
- Yang Q, Phillips CL, Melehan KL, et al. Effects of short-term CPAP withdrawal on neurobehavioral performance in patients with obstructive sleep apnea. Sleep 2006;29:545-52. [Crossref] [PubMed]
- Young LR, Taxin ZH, Norman RG, et al. Response to CPAP withdrawal in patients with mild versus severe obstructive sleep apnea/hypopnea syndrome. Sleep 2013;36:405-12. [Crossref] [PubMed]
- Turkington PM, Sircar M, Saralaya D, et al. Time course of changes in driving simulator performance with and without treatment in patients with sleep apnoea hypopnoea syndrome. Thorax 2004;59:56-9. [PubMed]
- Sforza E, Lugaresi E. Daytime sleepiness and nasal continuous positive airway pressure therapy in obstructive sleep apnea syndrome patients: effects of chronic treatment and 1-night therapy withdrawal. Sleep 1995;18:195-201. [PubMed]
- Filtness AJ, Reyner LA, Horne JA. One night's CPAP withdrawal in otherwise compliant OSA patients: marked driving impairment but good awareness of increased sleepiness. Sleep Breath 2012;16:865-71. [Crossref] [PubMed]
- Phillips CL, Yee B, Yang Q, et al. Effects of continuous positive airway pressure treatment and withdrawal in patients with obstructive sleep apnea on arterial stiffness and central BP. Chest 2008;134:94-100. [Crossref] [PubMed]
- Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:1162-8. [Crossref] [PubMed]
- Bonsignore MR, Parati G, Insalaco G, et al. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2002;166:279-86. [Crossref] [PubMed]
- Ip MS, Tse HF, Lam B, et al. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 2004;169:348-53. [Crossref] [PubMed]
- Marrone O, Salvaggio A, Bonsignore MR, et al. Blood pressure responsiveness to obstructive events during sleep after chronic CPAP. Eur Respir J 2003;21:509-14. [Crossref] [PubMed]
- Jun JC, Unnikrishnan D, Schneider H, et al. Effect of Acute Intermittent CPAP Depressurization during Sleep in Obese Patients. PLoS One 2016;11:e0146606. [Crossref] [PubMed]
- Chopra S, Rathore A, Younas H, et al. Obstructive Sleep Apnea Dynamically Increases Nocturnal Plasma Free Fatty Acids, Glucose, and Cortisol during Sleep. J Clin Endocrinol Metab 2017;102:3172-81. [Crossref] [PubMed]
- Boerner B, Tini GM, Fachinger P, et al. Significant improvement of olfactory performance in sleep apnea patients after three months of nasal CPAP therapy - Observational study and randomized trial. PLoS One 2017;12:e0171087. [Crossref] [PubMed]
- Williams SC, Rogers NL, Marshall NS, et al. The effect of modafinil following acute CPAP withdrawal: a preliminary study. Sleep Breath 2008;12:359-64. [Crossref] [PubMed]
- Williams SC, Marshall NS, Kennerson M, et al. Modafinil effects during acute continuous positive airway pressure withdrawal: a randomized crossover double-blind placebo-controlled trial. Am J Respir Crit Care Med 2010;181:825-31. [Crossref] [PubMed]
- Wang D, Bai XX, Williams SC, et al. Modafinil Increases Awake EEG Activation and Improves Performance in Obstructive Sleep Apnea during Continuous Positive Airway Pressure Withdrawal. Sleep 2015;38:1297-303. [Crossref] [PubMed]
- Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014;370:139-49. [Crossref] [PubMed]


