Clarithromycin mitigates radiation pneumonitis in patients with lung cancer treated with stereotactic body radiotherapy
Introduction
Radiation pneumonitis is a critical pulmonary toxicity following irradiation of the lung and can be fatal. Corticosteroids are considered the first-line treatment for radiation pneumonitis despite a lack of data. They are ineffective to patients with severe radiation pneumonitis (1). Although stereotactic body radiotherapy (SBRT) for lung cancer is relatively safe, radiation pneumonitis remains a significant concern, particularly in patients with idiopathic interstitial pneumonias (IIPs).
Radiation pneumonitis is characterized by interstitial pneumonia [i]. Severe radiation pneumonitis causes extensive involvement outside the irradiated tissue, sometimes resulting in bilateral lymphocytic alveolitis, suggesting an immunologically mediated process. Neutrophilic infiltration has been shown to have an important role in the development of radiation pneumonitis (2-5). Although it is infrequent, severe radiation pneumonitis can occur early after SBRT and progress rapidly (6). Furthermore, severe radiation pneumonitis in patients with IIPs may have certain etiological features in common with acute exacerbations of IIPs (7-12).
Macrolides are antibiotics and often prescribed for community-acquired respiratory infections. In addition to their antibiotic activity, low dose macrolides have immunomodulatory effects due to neutrophil inactivation (13,14). Over the past 30 years, macrolides such as erythromycin, clarithromycin (CAM), and azithromycin have been used to treat inflammatory respiratory disease such as diffuse panbronchiolitis (15) and cystic fibrosis (16). Moreover, macrolide maintenance therapy has been shown to improve pulmonary function and/or frequency of exacerbations in patients with chronic obstructive pulmonary disease (COPD) (17), chronic sinusitis, asthma (18), and bronchiectasis (19). Macrolides have also been shown to be effective in treating chronic fibrosing interstitial pneumonia (20), idiopathic pulmonary fibrosis (IPF) (21,22), and cryptogenic and radiation-related organizing pneumonia (23,24). In addition, CAM is reported to attenuate RP in mice (25).
The adverse effects of macrolides are rare and generally self-limited when these medications are used at the low doses recommended for immunomodulation.
Based on these findings, since January 2014 we have administered CAM to patients suspected to be at high risk for developing radiation pneumonitis. In this study, we retrospectively investigated whether CAM could mitigate radiation pneumonitis in lung cancer patients following SBRT.
Methods
Patients
Among consecutive patients treated with SBRT in our hospital between February 2005 and April 2016, we retrospectively identified patients with lung cancer who were treated with SBRT, with a total dose of 40–60 Gy in 5–10 fractions. The patients included those staged as cT1-4N0M0 using the 7th lung cancer TNM classification and staging system and those with postoperative local recurrence without nodal or distant metastasis. The patients were excluded who were lost to follow-up within 6 months. Although biopsy was offered to most patients, biopsy results were not available for all patients, due to patient refusal, or technical or clinical difficulties. For patients without histological confirmation of disease, a clinical diagnosis of lung cancer was made by a lung cancer review board based on clinical information such as an increase in the maximum standardized uptake value on [18F] fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), successive enlargements on computed tomography (CT) images, and elevated tumor marker levels. IIPs were diagnosed by two pulmonologists with expertise in IIPs and a diagnostic radiologist (26). All patients provided written informed consent. The study was approved by the Ofuna Chuo Hospital Review Board (No. 2016-09).
SBRT
We previously described our SBRT methods in detail (27). For the treatment-planning CT, the patient was immobilized with a vacuum pillow and abdominal corset that restricts respiratory motion within 1 cm, and a long-scan-time CT was performed to directly visualize the internal target volume. The planning target volume was determined by adding a margin of 6–8 mm to the internal target volume. For SBRT delivery, dynamic conformal multiple arc therapy was used until January 2012; subsequently, non-coplanar volumetric modulated arc therapy was used. Until April 2011, a total dose of 50 Gy was used for peripherally located lesions and 40 Gy was used for centrally located lesions in 5 fractions at 80% isodose line of the maximum dose. Beginning in May 2011, we used three total doses in 5 fractions at 60% isodose line of the maximum dose; 60 Gy for peripherally located lesions and non-adjacent to the chest wall, 50 Gy for peripherally located lesions adjacent to the chest wall and for centrally located lesions not including the main bronchus and/or main pulmonary artery within planning target volume, and 40 Gy for centrally located lesions including the main bronchus and/or main pulmonary artery within planning target volume.
CAM administration
Since January 2014, we have administered oral CAM for patients who had following high risk factors for radiation pneumonitis, using 200 mg/day once daily for 3 months from the start of SBRT. Pretreatment predictable high-risk factors included at least one of the following: IIPs or secondary interstitial pneumonia (12,28); elevated Krebs von den Lungen-6 (KL-6) (>500 U/mL) and/or surfactant protein D (SP-D) (>110 ng/mL) (29,30). CAM was also administered to patients with other risk factors at the physicians' discretion; risk factors included severely decreased pulmonary function, a history of thoracic irradiation, and severe steroid-dependent asthma. In addition, CAM was administered to patients with an onset of radiation pneumonitis ≥ grade 1 within 2.5 months after SBRT because these patients were regarded to have a high risk of radiation pneumonitis (8,9); patients were treated with 200 mg/day once daily for 3 months from the onset of radiation pneumonitis. Before administration of CAM, patients were informed that CAM is an antibiotic and also has immunomodulatory effects; it has been used to treat inflammatory respiratory diseases with neutrophilic inflammation; radiation pneumonitis has similar pathology to them.
Follow up
During the first 6 months after SBRT, all patients were monitored monthly with interviews, laboratory data review, and chest X-ray examination. Chest imaging follow-up included high-resolution CT scans performed at 1 and 3 months after SBRT and thereafter at 3-month intervals during the first 2 years, even in the absence of clinical symptoms. Subsequently, follow-up interviews, laboratory data review, and CT scans were obtained at 4–6 months intervals. 18F-FDG PET/CT was performed to assess the local recurrence and to detect distant metastases at approximately one year after SBRT and when recurrences were suspected. Toxicities were evaluated by radiation oncologists, respirologists and diagnostic radiologists. Toxicity was graded using the common terminology criteria for adverse events (CTCAE) Version 4.0.
Radiation pneumonitis after SBRT, excluding infection, was defined as a graphical change around the planning target volume with or without new or worsening respiratory symptom such as dry cough, shortness of breath and fever. Infectious pneumonitis was defined pneumonitis which proven bacterial infection by germ culture, and patients recovered by antibiotic. In CTCAE Version 4.0, radiation pneumonitis was graded as follows: grade 1, asymptomatic radiation pneumonitis observed on diagnostic imaging and not requiring intervention; grade 2, symptomatic radiation pneumonitis requiring medical intervention; grade 3, severe symptoms limiting patient self-care and requiring oxygen; grade 4, life-threatening respiratory compromise requiring urgent intervention; and grade 5, death from radiation pneumonitis. Steroid was administered when patients had severe respiratory symptoms. Outpatients requiring corticosteroids were graded as grade 2, and patients in hospitalization were graded as grade 3 regardless of oxygen administration.
Analysis and statistics
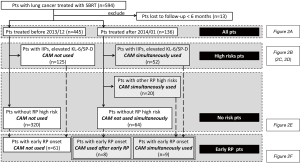
Figure 1 shows the analysis flow chart. First, we analyzed the incidence of radiation pneumonitis in all patients in each era. Second, we extracted patients with and without the pretreatment predictable high-risk factors for radiation pneumonitis and analyzed each of these groups accordingly. Third, we extracted patients with early graphical onset of radiation pneumonitis and analyzed accordingly, as these patients were regarded to have a high risk of radiation pneumonitis (8,9). Specifically, patients treated with CAM in the CAM-administration era (CAM-era) included those who received CAM from the start of SBRT due to the presence of pretreatment predictable high-risk factors and those who received CAM after early graphical onset of radiation pneumonitis was recognized.
Patient characteristics were compared using the Mann-Whitney test and the Chi-square test. Follow-up was defined as starting from the date of the first SBRT to determine median follow-up and time-to-event. Logistic regression analysis was used to assess correlations between characteristic factors and radiation pneumonitis using both univariate and multivariate models. Univariate factors with P<0.15 were included in the multivariate analysis. Important factors directly related to this study’s main objective were also included, regardless of P value. When the correlation coefficient (r) between factors exceeded 0.9, the more clinically important factor was included. When the number of events was not enough to evaluate candidate factors, some factors were excluded after considering clinical importance and correlation coefficient. Values of P<0.05 were considered statistically significant. Data were analyzed with JMP® 11 (SAS Institute Inc., Cary, NC, USA).
Results
Eligible patients
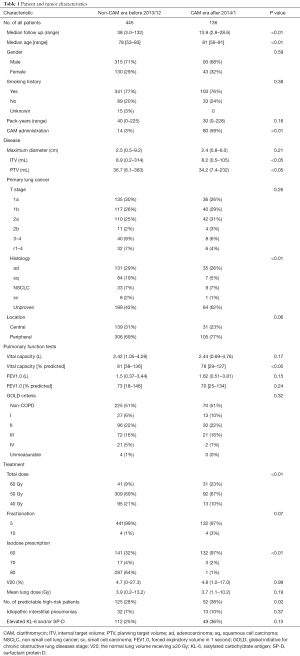
Between February 2005 and April 2016, 594 patients received SBRT for lung cancer with a total dose of 40–60 Gy in 5–10 fractions. Among these, 13 patients were lost to follow-up within 6 months and were excluded. None of the excluded patients suffered from radiation pneumonitis during the follow-up. The remaining 581 patients were eligible for inclusion in the study. Patients were divided into two groups; the 445 patients treated with SBRT before December 2013 during the non-CAM-administration era (non-CAM-era) and the 136 patients treated with SBRT after January 2014 during CAM-era. Among 445 patients in non-CAM-era, 14 patients had already been administered for their comorbidities of COPD and chronic lower respiratory tract infection. No one was given CAM for mitigating RP. Among 136 patients in CAM-era, 80 patients were treated with CAM. CAM was administered to 52 patients with predictable high-risk factors. The other reason for CAM administration included history of irradiation in 10 patients, history of thoracic surgery in 3 patients, very severe emphysema (Global Initiative for Chronic Obstructive Lung Diseases stage III) in 5 patients, asthma with steroid administration in 2 patients, and rheumatoid arthritis with methotrexate in 1 patient. In addition, CAM was administered to 8 patients with an early onset of radiation pneumonitis. Baseline patient characteristics are shown in Table 1. Median follow-up durations for patients in the non-CAM-era and the CAM-era were 38.0 and 13.9 months, respectively.
Full table
Some baseline patient characteristics were significantly different between the two eras. Some differences were due to transition of treatment policy, including CAM administration, total dose, isodose prescription, and inclusion of high-risk patients. Internal target volume, planning target volume, age, histology, and % predicted vital capacity were statistically significantly different between the two eras, but the differences were too small to consider. In contrast, the volume of lung receiving ≥20 Gy (V20) and mean lung dose were similar between the two eras. Other characteristics were also similar between the two eras.
Rates of radiation pneumonitis
Figure 2 shows the numbers and rates of radiation pneumonitis by grades. Among all patients, the rates of radiation pneumonitis ≥ grade 2 and ≥ grade 3 were significantly lower in the CAM-era than in the non-CAM-era (grade ≥2, 16% vs. 9.6%, P=0.047; grade ≥3, 3.8% vs. 0.73%, P=0.037) (Figure 2A).
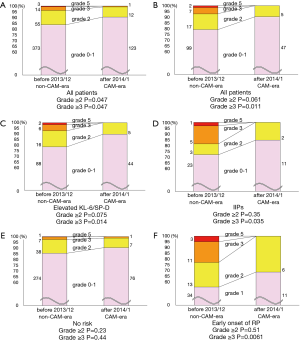
We then evaluated only those patients with pretreatment predictable high-risk factors for developing radiation pneumonitis. The numbers of patients in the non-CAM-era and the CAM-era were 125 and 52, respectively. The rate of radiation pneumonitis ≥ grade 3 was significantly lower in the CAM-era, and the rate of grade ≥2 had a lower tendency in the CAM-era (grade ≥3, 7.2% vs. 0%, P=0.011; grade ≥2, 21% vs. 9.6%, P=0.061) (Figure 2B). Specifically, patients with elevated KL-6 and/or SP-D showed a tendency toward lower rates of radiation pneumonitis ≥ grade 2 in the CAM-era and had significantly lower rates of radiation pneumonitis ≥ grade 3 (Figure 2C). Patients with IIPs had significantly lower rates of radiation pneumonitis ≥ grade 3 in the CAM-era (Figure 2D). Additionally, no patients with IIPs suffered from radiation pneumonitis ≥ grade 3 in the CAM-era. In contrast, among patients with no identified risk factors, there were no differences in the rates of radiation pneumonitis between the two eras (Figure 2E).
We next evaluated patients with early onset radiation pneumonitis that appeared radiographically within 2.5 months after SBRT. The rates of early onset radiation pneumonitis were similar in the non-CAM-era and the CAM-era: 14% vs. 13%, respectively, P=0.68. However, the severity of radiation pneumonitis was significantly different between the two eras. No patient in the CAM-era developed grade ≥3 radiation pneumonitis, but 14 patients (23%) developed radiation pneumonitis grade ≥3 in the non-CAM-era (61 patients) (Figure 2F).
Univariate and multivariate analyses
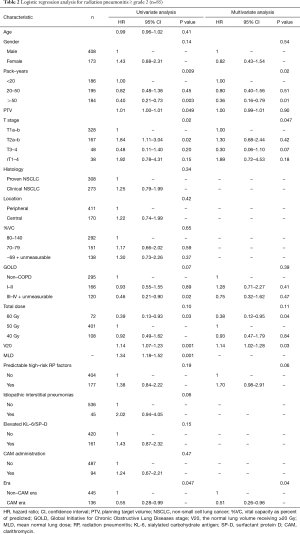
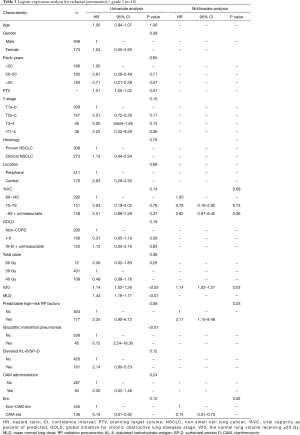
Univariate analysis revealed that pack-years, planning target volume, T stage, V20, mean lung dose, and non-CAM-era were significant predictors of radiation pneumonitis ≥ grade 2 (Table 2). Mean lung dose was excluded from the multivariate analysis because the correlation coefficient (r) between V20 and mean lung dose exceeded 0.9. In the multivariate analysis, pack-years, T stage, V20, and non-CAM-era remained significant risk factors. The pretreatment predictable high-risk factors for radiation pneumonitis showed a tendency toward radiation pneumonitis.
Full table
In patients with radiation pneumonitis ≥ grade 3 (Table 3), univariate analysis revealed that V20, mean lung dose, and IIPs were significant predictive factors. planning target volume was excluded, as was mean lung dose, because the number of events (n=18) was small. However, the dosimetry factor V20, a clinically important factor for radiation pneumonitis, was included. In the multivariate analysis, V20, the pretreatment predictable high-risk factors, and non-CAM-era remained significant.
Full table
Discussion
Radiation pneumonitis is one of the most critical pulmonary toxicities following SBRT for lung tumors. Many previous radiation pneumonitis studies have investigated risk factors (12,28-31), dose-volumetric analyses (32,33), and prophylactic drugs (34,35).
IIPs are one risk factor for the development of radiation pneumonitis (30,31). Several reports have suggested that radiation pneumonitis ≥ grade 3 in patients with IIPs may have certain etiological features in common with acute exacerbations of IIPs (7-12). In addition, KL-6 and SP-D, which are serum marker for IIPs, play a role in detecting patients who are at high risk for radiation pneumonitis (29,30) and in monitoring the severity of radiation pneumonitis (36). In patients with IIPs, severe radiation pneumonitis starts very early after SBRT and is progressive and often fatal (8,9). In addition, according to a report on patients who suffer from grade 5 radiation pneumonitis after lung SBRT, more than half had usual interstitial pneumonia (UIP) pattern on CT (37). Based on these reports, IPF patients were contraindicated in previous prospective SBRT studies (38,39) and IPF has been regarded as a relative contraindication to SBRT in clinical practice. Despite this, we have carefully treated IIPs patients with SBRT at our institution after thorough informed consent.
AEs of IIPs often follows various lung cancer treatments, including radiotherapy (40), surgery (41), and antineoplastic agents (42). For lung cancer patients treated with surgery, prophylactic macrolides have been empirically administered in 38 of 220 (17%) Japanese institutions (43) although the efficacy of macrolide therapy has been uncertain. We thus investigated the immunomodulatory effect of macrolides in patients at high risk for developing radiation pneumonitis. We found that administration of CAM decreased the rate of radiation pneumonitis and improved radiation pneumonitis severity. This is the first study to demonstrate the efficacy of prophylactic CAM administration for mitigating radiation pneumonitis.
Mechanisms of radiation pneumonitis, and the immunomodulatory effects of macrolides
Although the specific mechanisms underlying radiation pneumonitis and the immunomodulatory effects of macrolides remain uncertain, some evidence suggests that macrolides may be an effective way to mitigate radiation pneumonitis.
Radiation pneumonitis is characterized by interstitial pneumonia (44). The early histopathologic finding after radiotherapy is described as diffuse alveolar damage (4). This includes edema of the alveolar walls due to increased vascular permeability and exudation of proteins into the alveolar space. Inflammatory cell infiltration is generally present. Activated macrophages produce a variety of cytokines with mitogenic or chemotactic properties that lead to neutrophil and lymphocyte recruitment (4). Irradiation to the lung significantly increased neutrophils and lymphocytes in bronchoalveolar lavage fluid in murine models and in humans (2,3). A genetic variant of interleukin-8 (IL-8) (rs4073), which is associated with increased secretion of IL-8, was found to increase radiation pneumonitis risk 3-fold (5).
Macrolides have immunomodulatory effects. These inhibit neutrophil activation and mobilization, accelerate neutrophil apoptosis, afford cytoprotection, and decrease activation of nuclear transcription factors mediated by various cytokines. IL-8 is one of the key cytokines and a major neutrophil chemoattractant, and decrease the number of neutrophils recruited in the airway (13). Macrolides significantly reduce neutrophil counts in bronchoalveolar lavage fluid by suppressing IL-8 production in patients with diffuse panbronchiolitis (45). In vitro, EM and CAM suppressed IL-8 messenger RNA (mRNA) expression as well as protein levels in normal human bronchial epithelial cells and transformed bronchial epithelial cells (46). In addition, CAM ameliorates the deleterious effects of thoracic irradiation by reducing respiratory inflammation, oxidative damage, and fibrosis (25). CAM can shift cells toward a quiescent stage (G0/G1) affording cytoprotection as inflammation and oxidative stress is the most damaging to growing cells (47-49).
Several reports have demonstrated that macrolides were effective, or facilitated the efficacy of other therapies, in the treatment of IIPs (20-22) and organizing pneumonia (23,24). Macrolide therapy combined with other agents significantly decreased the incidence of acute exacerbations in patients with IPF (21). Macrolides combined with high-dose corticosteroids may also be effective in mitigating rapid progression of respiratory failure in patients with acute exacerbations of IPF (22), and also decreased mortality rate significantly in patients with acute exacerbation of chronic fibrosing interstitial pneumonia (20). In addition, macrolides were effective in treating cryptogenic organizing pneumonia (23,24) and radiation-related organizing pneumonia (24).
Risk factors for radiation pneumonitis
In this study, we considered IIPs, pre-treatment elevation of KL-6 and/or SP-D, and early onset of radiation pneumonitis as risk factors for severe radiation pneumonitis (8,9,12,28-30), and administered CAM to patients with those risks. Dose-volumetric factors related to the lung were not included as high-risk factors in the current study, although they are well known to be risk factors for radiation pneumonitis (32,33). In these studies, grade 2 radiation pneumonitis was targeted as the primary event. Grade 2 radiation pneumonitis is defined as symptomatic dyspnea that limits the activities of daily living and may require initiation or increase of corticosteroids; it is often mild and remits spontaneously in patients treated with SBRT. In contrast, grade 3 radiation pneumonitis, in which we are primarily interested, is severe and may worsen to a higher grade radiation pneumonitis. We previously analyzed dose-volumetric factors among patients with grade 0–1, grade 2, and grade 3 radiation pneumonitis (50). We found that V5–30 and mean lung dose in patients with grade 2 radiation pneumonitis were significantly higher than in patients with grade 0–1. However, V5–30 and mean lung dose in patients with grade 3 radiation pneumonitis were as low as in patients with grade 0–1 radiation pneumonitis. These results suggested that severe radiation pneumonitis grade is independent of dose-volumetric factors, may have a unique etiology, and may occur in patients who are more susceptible to acute radiation injury, even when safer dosimetry values are used. In other words, grade 3 radiation pneumonitis may not always occur as a progression from grade 2 radiation pneumonitis. Therefore, we did not prescribe CAM to patients with higher dose-volumetric values.
Multivariate analysis revealed that the dose-volumetric factor mean lung dose was a significant risk factor for both radiation pneumonitis ≥ grade 2 and ≥ grade 3, as well as IIPs and elevated KL-6 and/or SP-D. Future prospective clinical studies are required to validate whether radiation pneumonitis can be mitigated by CAM in patients with high dose-volumetric factors and in patients with IIPs and elevated KL-6 and/or SP-D. In addition, we should pay attention to the other potential risk factors such as the use of molecular targeted agents and immune checkpoint inhibitors.
Radiation pneumonitis occurs more often when patients with locally advanced lung cancer are treated with conventionally fractioned chemoradiotherapy because radiation fields are larger and concurrent chemotherapy enhances the risk of severe radiation pneumonitis. If macrolides are truly effective to mitigate radiation pneumonitis, it will contribute to safer treatment in advanced lung cancer patients.
Limitation
Although CAM seems to mitigate both mild and severe radiation pneumonitis, the optimal CAM dosage, duration and indication, and the appropriate treatment candidates, remain unknown. In the single patient in our study who suffered from grade 3 radiation pneumonitis in spite of CAM administration, CAM was stopped 3 months after SBRT when a small ground-glass opacity (grade 1) appeared. The patient subsequently deteriorated and ultimately required continuous oxygen therapy (grade 3). Because CAM is safely used as a long-term anti-inflammatory therapy in patients with chronic inflammatory lung diseases, longer administration may have been tolerated in this patient, and may have prevented his subsequent decline.
This study has several limitations. First, this was a retrospective study, performed at a single-institution, and divided into two time periods. The patients treated in the non-CAM-era represent an older cohort who may have undergone older treatment modalities. SBRT methods and dose prescription have slightly changed. However, comparison of patient and tumor characteristics between the two eras revealed little difference in factors. Second, the indication for CAM treatment in this study was not thoroughly consistent. Third, median follow-up time in the CAM-era was shorter at 13.5 vs. 38 months. The length of follow-up for some of the patients in the CAM-era group may be too short to capture some radiation pneumonitis events. Nevertheless, we found that CAM appears to mitigate radiation pneumonitis, a finding that should be confirmed in larger prospective studies.
Conclusions
CAM mitigated radiation pneumonitis in patients with lung cancer treated with SBRT. The efficacy of CAM should be confirmed in larger prospective studies.
Acknowledgements
Dr.Takeda reports grants from Varian research grant, grants from Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science, during the conduct of the study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ofuna Chuo Hospital Review Board (No. 2016-09). All patients provided written informed consent.
References
- Inoue A, Kunitoh H, Sekine I, et al. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys 2001;49:649-55. [Crossref] [PubMed]
- Rosiello RA, Merrill WW, Rockwell S, et al. Radiation pneumonitis. Bronchoalveolar lavage assessment and modulation by a recombinant cytokine. Am Rev Respir Dis 1993;148:1671-6. [Crossref] [PubMed]
- Bjermer L, Franzen L, Littbrand B, et al. Effects of smoking and irradiated volume on inflammatory response in the lung of irradiated breast cancer patients evaluated with bronchoalveolar lavage. Cancer Res 1990;50:2027-30. [PubMed]
- Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys 2006;66:1281-93. [Crossref] [PubMed]
- Hildebrandt MA, Komaki R, Liao Z, et al. Genetic variants in inflammation-related genes are associated with radiation-induced toxicity following treatment for non-small cell lung cancer. PLoS One 2010;5:e12402. [Crossref] [PubMed]
- Morgan GW, Breit SN. Radiation and the lung: a reevaluation of the mechanisms mediating pulmonary injury. Int J Radiat Oncol Biol Phys 1995;31:361-9. [Crossref] [PubMed]
- Sugiura H, Takeda A, Hoshi T, et al. Acute exacerbation of usual interstitial pneumonia after resection of lung cancer. Ann Thorac Surg 2012;93:937-43. [Crossref] [PubMed]
- Takeda A, Ohashi T, Kunieda E, et al. Early graphical appearance of radiation pneumonitis correlates with the severity of radiation pneumonitis after stereotactic body radiotherapy (SBRT) in patients with lung tumors. Int J Radiat Oncol Biol Phys 2010;77:685-90. [Crossref] [PubMed]
- Sekine I, Sumi M, Ito Y, et al. Retrospective analysis of steroid therapy for radiation-induced lung injury in lung cancer patients. Radiother Oncol 2006;80:93-7. [Crossref] [PubMed]
- Sanuki N, Ono A, Komatsu E, et al. Association of computed tomography-detected pulmonary interstitial changes with severe radiation pneumonitis for patients treated with thoracic radiotherapy. J Radiat Res 2012;53:110-6. [Crossref] [PubMed]
- Yamaguchi S, Ohguri T, Ide S, et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: The potential risk of extensive radiation pneumonitis. Lung Cancer 2013;82:260-5. [Crossref] [PubMed]
- Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol 2015;10:116-25. [Crossref] [PubMed]
- Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacol Ther 2008;117:393-405. [Crossref] [PubMed]
- Zarogoulidis P, Papanas N, Kioumis I, et al. Macrolides: from in vitro anti-inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. Eur J Clin Pharmacol 2012;68:479-503. [Crossref] [PubMed]
- Kudoh S, Azuma A, Yamamoto M, et al. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 1998;157:1829-32. [Crossref] [PubMed]
- Jaffé A, Francis J, Rosenthal M, et al. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet 1998;351:420. [Crossref] [PubMed]
- Yamaya M, Azuma A, Tanaka H, et al. Inhibitory effects of macrolide antibiotics on exacerbations and hospitalization in chronic obstructive pulmonary disease in Japan: a retrospective multicenter analysis. J Am Geriatr Soc 2008;56:1358-60. [Crossref] [PubMed]
- Amayasu H, Yoshida S, Ebana S, et al. Clarithromycin suppresses bronchial hyperresponsiveness associated with eosinophilic inflammation in patients with asthma. Ann Allergy Asthma Immunol 2000;84:594-8. [Crossref] [PubMed]
- Tsang KW, Ho PI, Chan KN, et al. A pilot study of low-dose erythromycin in bronchiectasis. Eur Respir J 1999;13:361-4. [Crossref] [PubMed]
- Kawamura K, Ichikado K, Suga M. Efficacy of azithromycin for treatment of acute exacerbation of chronic fibrosing interstitial pneumonia: a prospective, open-label study with historical controls. Respiration 2014;87:478-84. [Crossref] [PubMed]
- Kuse N, Abe S, Hayashi H, et al. Long-term efficacy of macrolide treatment in idiopathic pulmonary fibrosis: a retrospective analysis. Sarcoidosis Vasc Diffuse Lung Dis 2016;33:242-6. [PubMed]
- Oda K, Yatera K, Fujino Y, et al. Efficacy of concurrent treatments in idiopathic pulmonary fibrosis patients with a rapid progression of respiratory failure: an analysis of a national administrative database in Japan. BMC Pulm Med 2016;16:91. [Crossref] [PubMed]
- Pathak V, Kuhn JM, Durham C, et al. Macrolide use leads to clinical and radiological improvement in patients with cryptogenic organizing pneumonia. Ann Am Thorac Soc 2014;11:87-91. [Crossref] [PubMed]
- Stover DE, Mangino D. Macrolides: a treatment alternative for bronchiolitis obliterans organizing pneumonia? Chest 2005;128:3611-7. [Crossref] [PubMed]
- Lee SJ, Yi CO, Heo RW, et al. Clarithromycin attenuates radiation-induced lung injury in mice. PLoS One 2015;10:e0131671. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Takeda A, Kunieda E, Sanuki N, et al. Dose distribution analysis in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. Int J Radiat Oncol Biol Phys 2009;74:363-9. [Crossref] [PubMed]
- Bahig H, Filion E, Vu T, et al. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol 2016;6:367-74. [Crossref] [PubMed]
- Iwata H, Shibamoto Y, Baba F, et al. Correlation between the serum KL-6 level and the grade of radiation pneumonitis after stereotactic body radiotherapy for stage I lung cancer or small lung metastasis. Radiother Oncol 2011;101:267-70. [Crossref] [PubMed]
- Yamashita H, Kobayashi-Shibata S, Terahara A, et al. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat Oncol 2010;5:32. [Crossref] [PubMed]
- Lee YH, Kim YS, Lee SN, et al. Interstitial lung change in pre-radiation therapy computed tomography is a risk factor for severe radiation pneumonitis. Cancer Res Treat 2015;47:676-86. [Crossref] [PubMed]
- Guckenberger M, Baier K, Polat B, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol 2010;97:65-70. [Crossref] [PubMed]
- Harder EM, Park HS, Chen ZJ, et al. Pulmonary dose-volume predictors of radiation pneumonitis following stereotactic body radiation therapy. Pract Radiat Oncol 2016;6:e353-9. [Crossref] [PubMed]
- Harder EM, Park HS, Nath SK, et al. Angiotensin-converting enzyme inhibitors decrease the risk of radiation pneumonitis after stereotactic body radiation therapy. Pract Radiat Oncol 2015;5:e643-9. [Crossref] [PubMed]
- Alite F, Balasubramanian N, Adams W, et al. Decreased risk of radiation pneumonitis with coincident concurrent use of angiotensin-converting enzyme inhibitors in patients receiving lung stereotactic body radiation therapy. Am J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Hara R, Itami J, Komiyama T, et al. Serum levels of KL-6 for predicting the occurrence of radiation pneumonitis after stereotactic radiotherapy for lung tumors. Chest 2004;125:340-4. [Crossref] [PubMed]
- Onishi H, Marino K, Terahara A, et al. Case series study of 26 patients who developed fatal radiation pneumonitis (RP) after stereotactic body radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2009;75:S62. [Crossref]
- Onimaru R, Shirato H, Shibata T, et al. Phase I study of stereotactic body radiation therapy for peripheral T2N0M0 non-small cell lung cancer with PTV<100 cc using a continual reassessment method (JCOG0702). Radiother Oncol 2015;116:276-80. [Crossref] [PubMed]
- Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys 2015;93:989-96. [Crossref] [PubMed]
- Takeda A, Enomoto T, Sanuki N, et al. Acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by hypofractionated stereotactic body radiotherapy in a patient with primary lung cancer and slightly focal honeycombing. Radiat Med 2008;26:504-7. [Crossref] [PubMed]
- Sato T, Teramukai S, Kondo H, et al. Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014;147:1604-11.e3. [Crossref] [PubMed]
- Chen YJ, Chen LX, Han MX, et al. The efficacy and safety of chemotherapy in patients with nonsmall cell lung cancer and interstitial lung disease: A PRISMA-compliant bayesian meta-analysis and systematic review. Medicine (Baltimore) 2015;94:e1451. [Crossref] [PubMed]
- Miyamoto A, Kishi K, Yoshimura K. A nationwide survey concerning lung surgery for lung cancer associated with idiopathic interstitial pneumonia. Nihon Kokyuki Gakkai Zasshi 2011;49:148-50. [PubMed]
- Brush J, Lipnick SL, Phillips T, et al. Molecular mechanisms of late normal tissue injury. Semin Radiat Oncol 2007;17:121-30. [Crossref] [PubMed]
- Oishi K, Sonoda F, Kobayashi S, et al. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun 1994;62:4145-52. [PubMed]
- Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin modulates IL-8 expression in normal and inflamed human bronchial epithelial cells. Am J Respir Crit Care Med 1997;156:266-71. [Crossref] [PubMed]
- Morris GM. Review article: effects of radiation on the cell proliferation kinetics of epithelial tissues--therapeutic implications. Br J Radiol 1996;69:795-803. [Crossref] [PubMed]
- Lee TK, Stupans I. Radioprotection: the non-steroidal anti-inflammatory drugs (NSAIDs) and prostaglandins. J Pharm Pharmacol 2002;54:1435-45. [Crossref] [PubMed]
- Shinkai M, Tamaoki J, Kobayashi H, et al. Clarithromycin delays progression of bronchial epithelial cells from G1 phase to S phase and delays cell growth via extracellular signal-regulated protein kinase suppression. Antimicrob Agents Chemother 2006;50:1738-44. [Crossref] [PubMed]
- Takeda A, Ohashi T, Kunieda E, et al. Comparison of clinical, tumour-related and dosimetric factors in grade 0-1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. Br J Radiol 2012;85:636-42. [Crossref] [PubMed]


