Perspectives on oligometastasis: challenges and opportunities
The concept that patients with only a few metastases from a malignant tumor can potentially be cured was developed in 1995 and termed “Oligo” (few) metastasis, describing an intermediate stage between localized and metastasized cancer (1). This concept is based on the hypothesis that there is a distinct stage of limited metastatic capability of the primary tumor (2): metastatic spread that behaves hierarchical in time and number opens the therapeutic window for a cure at an early, an “oligometastatic” stage.
Based on this concept, physicians have started treating these patients with locally ablative therapies in a curative intention. For instance, resection of colorectal liver metastases has led to long-term survival of patients (3). Similarly for non-small cell lung cancer (NSCLC), data suggests that long-term survival may be achieved for a subset of oligometastatic patients if treated radically (4). However, details of treatment concepts vary widely and only few studies have prospectively investigated the optimal treatment strategies for such patients. For instance, the definition of how many metastases are in fact “oligo—a few” remains controversial. Illustrating this point, 20 clinical studies examined by Reyes and Pienta on oligometastatic lung cancer used 17 different definitions and each study performed a different treatment pattern (5). Nevertheless, according to a survey conducted among over 1,000 radiation oncologists in over 40 countries in 2017, a large majority (83%) is treating patients in an oligometastatic setting with stereotactic body radiation therapy (SBRT), also referred to as stereotactic ablative radiotherapy (SABR) and 59% of the remaining physicians plan to implement this treatment soon (6).
In the following, we will illustrate the current status of oligometastasis and future strategies from different perspectives.
Guidelines perspective
In NSCLC, the oligometastatic stage has been implemented into the 8th TNM-system (7), as well as the National Comprehensive Cancer Network (NCCN) guidelines (NCCN 3, 2017). Stage IVa M1b is defined as a single extrathoracic metastasis, where local treatment with radiotherapy or surgical resection is recommended. The ESMO guideline gives a less strong recommendation: For patients with one to three synchronous metastases, inclusion of patients into clinical trials for locally ablative treatment is recommended due to lack of high-level evidence. The exception is a solitary brain metastasis, for which stereotactic radiosurgery (SRS) or resection is recommended. In patients with activating driver mutations, only a level IVB recommendation is given for SBRT in the oligometastatic setting, because only retrospective data is currently available (8,9).
Prospective clinical trials
Despite being recommended in guidelines, the evidence for a patient benefit from local treatment in an oligometastatic stage is low and based on few studies.
Level I evidence for a survival benefit of locally ablative treatment exists for limited brain metastases: Andrews and colleagues published the results of the RTOG9508 phase III randomized controlled trial (RCT) in 2004 in which the group investigated the effect of adding a stereotactic boost to whole brain radiotherapy in patients with three or fewer brain metastases from any primary tumor. For patients with a single metastasis, a median overall survival (OS) benefit of 1.6 months was reported (10).
For unresectable colorectal hepatic metastases, a randomized phase II study (CLOCC-trial) evaluated OS for 119 patients without extrahepatic disease comparing systemic treatment alone with systemic treatment plus locally ablative treatment using radio-frequency ablation (11). In 2017, the long-term follow-up data were published showing a statistically significant OS benefit (HR 0.58, P=0.01) at almost 10 years follow-up for patients in the experimental arm. The OS-rate at 8 years was 35.9% vs. 8.9% for the combined therapy arm and the systemic treatment only arm, respectively (12).
For oligometastatic NSCLC, two prospective randomized studies are available: Gomez and colleagues have conducted a phase II RCT investigating the effect of local consolidation therapy after first line systemic treatment of oligometastatic (three or fewer lesions) NSCLC on progression free survival (PFS). The study was terminated early, because the group of 25 patients receiving locally consolidative treatment showed a much better PFS compared to the 24 patients treated with maintenance systemic treatment, only (11.9 vs. 3.9 months). Importantly, systemic progression was delayed in patients that received locally consolidative treatment suggesting that the benefit of such local treatment may reach beyond the local treatment site. The local consolidative therapy included surgery, radiotherapy, or both. Radiotherapy was the preferred treatment regimen: 96% of patients randomized in the local intervention arm received some form of radiotherapy, 48% received SBRT. Notably, no grade 4 or 5 toxicities were reported (13).
Iyengar and colleagues conducted a single institution phase II RCT comparing the PFS of NSCLC stage IV (up to five metastases) patients treated either with ablative radiotherapy followed by maintenance chemotherapy or with maintenance chemotherapy only. Of note, the authors defined the oligometastatic setting based on imaging performed after induction chemotherapy. All patients received maintenance chemotherapy either alone or in combination with SBRT. Furthermore, the authors excluded all patients, whose tumors harbored targetable mutations (Gomez et al. included such patients). This trial was small (n=29) as well and was likewise stopped early due to favorable results in the group treated with both, ablative radiotherapy and systemic treatment (9.7 vs. 3.5 months PFS, P=0.01). Toxicities were comparable in both groups and no in-field failures were observed in the group treated with ablative radiotherapy (14).
In summary, these two small prospective trials appear similar from a design perspective but large differences exist: Gomez et al. defined oligometastatic disease before first-line systemic treatment, whereas Iyengar et al. defined oligometastatic disease after first-line systemic treatment. The type of local therapy differed as well: While Iyengar et al. exclusively treated locally with SBRT, the Gomez study allowed all combinations of radiotherapy and surgery. Furthermore, Gomez et al. included patients with targetable tumor mutations, whereas Iyengar et al. excluded those. Gomez et al. included three and fewer metastases and Iyengar et al. included five and fewer.
Overall, evidence of a benefit of locally ablative treatments—especially SBRT—in the oligometastatic setting for NSCLC patients is evolving, however, randomized phase III trials are yet to be conducted. The current lack of evidence-based recommendations for patient selection and optimal combination of local and systemic treatment options is considered a relevant risk and marks a concern for broad clinical implementation.
Overview of clinical trials evaluating the local treatment component
Several prospective clinical trials are currently recruiting, which address different questions in the field of oligometastatic disease summarized in Table 1.
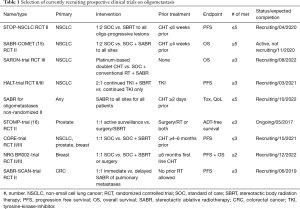
Full table
The STOP-NSCLC trial and the SABR-COMET-trial focus on treatment of oligoprogressive disease after systemic therapy for NSCLC compared to standard of care (SOC) treatment. The CORE-trial additionally investigates breast and prostate cancer in the oligoprogressive setting. The STOMP-trial and NCT002-trial pursue similar questions for prostate and breast cancer, respectively. In contrast, the SARON-trial is investigating the role of locally ablative treatments in the primary setting enrolling patients that have not received any tumor-therapy. The SABR-SCAN-trial aims to determine the optimal time for ablative treatment of pulmonary metastases from colorectal cancer (CRC) regarding PFS and patterns of progression comparing immediate with delayed treatment. The HALT-trial focuses on the role of local treatment for NSCLC patients with tumors that harbor a mutation targetable by a tyrosine-kinase-inhibitor (TKI) in the oligoprogressive setting. And finally, the SABR for oligometastases trial (NCT02933242) is enrolling patients with all tumor types focusing on the toxicity induced by such treatment and the quality of life (QoL) for patients receiving SABR.
Locally ablative treatment from a systemic perspective
As more patients are diagnosed with oligometastatic disease due to better imaging, loser definition of the term in general as well as longer survival, the correct identification of true oligometastatic disease is crucial. Biomarkers that avoid mistaking oligometastasis for systematic disease that was merely captured by imaging at a state of few metastases are urgently needed.
However, studies analyzing the molecular basis for oligometastasis are scarce: Lussier and colleagues analyzed miRNAs from resected pulmonary metastases of 63 patients with ≤5 pulmonary metastases and developed a rate-of-progression profile predicting the velocity of metastatic spread. The authors concluded that poly-metastasis that was captured at a state of few metastases and true oligometastasis are distinct entities on a molecular level (17).
Wong et al. analyzed miRNA from 17 primary tumors of any histology from patients with stage IV disease with five or fewer metastases treated within the group’s SBRT dose escalation study. They compared differential miRNA expression of patients that survived for more than 3 years to those who did not and developed a score for predicting survival time (18). The validation of this score is ongoing.
Gundem and colleagues proposed an evolution of metastases analyzing the genetic makeup of multiple metastases of ten prostate cancer patients (19). They found that a metastasis-to-metastasis spread seems common. This finding might account for the delay in systemic spread after locally ablative treatments mentioned above and should be further investigated to elucidate the biological rationale for treatment of oligometastasis.
Future applications perspective: SBRT and immunotherapy
The randomized phase III PACIFIC-trial recently showed an impressive gain of 11.2 months median PFS for stage III NSCLC patients receiving durvalumab, a PDL-1-antibody, as maintenance therapy after radiochemotherapy compared to placebo (20). Considering that these patients received radiotherapy within 42 days of randomization, this trial raises the question whether SBRT of oligometastasis combined with adjuvant immunotherapy might impact the survival benefit more drastically than previously imagined. Furthermore, it gives rise to the hypothesis that the reported PFS benefit of stage III patients might be extrapolated to oligometastatic stage IV patients that receive additional locally ablative treatments. Building on this premise, a phase II trial (SABR-PDL1) is currently recruiting patients with metastatic CRC, NSCLC or renal cell carcinoma (RCC) for a single arm study investigating the effects of atezolizumab (a PDL-1-antibody) combined with SABR to all but one lesion on PFS. Results are expected in 2020.
Perspective of opportunity: register studies
One possible solution to advance the heterogeneous field of oligometastasis are register trials. The above-mentioned guidelines have led to increasingly utilized locally ablative treatments of oligometastasis. The accumulating clinical data could be used to draw conclusions for safe and beneficial implementation of these guidelines. Register studies will assess patterns of care and outcome for patients and may bridge the gap between guidelines and lack of current evidence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378-82. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. [Crossref] [PubMed]
- Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget 2015;6:8491-524. [Crossref] [PubMed]
- Lewis SL, Porceddu S, Nakamura N, et al. Definitive Stereotactic Body Radiotherapy (SBRT) for Extracranial Oligometastases: An International Survey of >1000 Radiation Oncologists. Am J Clin Oncol 2017;40:418-22. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1-27. [Crossref] [PubMed]
- Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii27-39. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23:2619-26. [Crossref] [PubMed]
- Ruers T, Van Coevorden F, Punt CJ, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst 2017.109. [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Palma DA, Haasbeek CJ, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): study protocol for a randomized phase II trial. BMC Cancer 2012;12:305. [Crossref] [PubMed]
- Decaestecker K, De Meerleer G, Ameye F, et al. Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer 2014;14:671. [Crossref] [PubMed]
- Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs. PLoS One 2012;7:e50141. [Crossref] [PubMed]
- Wong AC, Watson SP, Pitroda SP, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 2016;122:2242-50. [Crossref] [PubMed]
- Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015;520:353-7. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]


