Solitary pulmonary nodule—a case of peripheral adenocarcinoma with rapid metastasis
Currently, an accepted definition of solitary pulmonary nodules (SPNs) is a single, clearly defined, radioopaque lesion in the lung surrounding entirely by air-containing lung tissue, in a diameter less than or equal to 3 cm, without atelectasis, hilar enlargement or pleural effusion (1,2). SPNs can be either benign or malignant. Their most common causes include infections and local inflammation. They often represent the lung malignancies, particularly small adenocarcinoma and bronchoalveolar carcinoma (Table 1). However, the distinguishment between benign and malignant SPNs and their treatment remain a hot and challenging research topic. This study describes a case with a SPN who had pleural effusion confirmed by chest computed tomography (CT) 23 days after anti-infection treatment, and pulmonary adencarcinoma was diagnosed via CT-guided lung biopsy. The progress from the initial diagnosis to the pleural metastasis is reported, and the whole body positron emission computed tomography (PET/CT) and pleural effusion EGFR mutation test results are included.
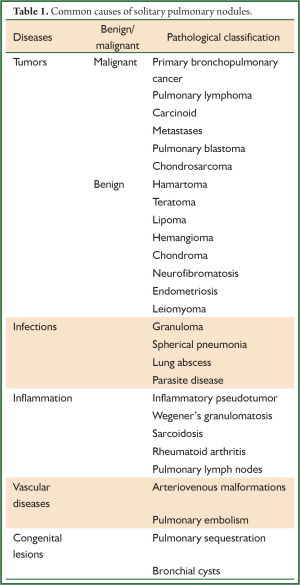
Full table
Case report
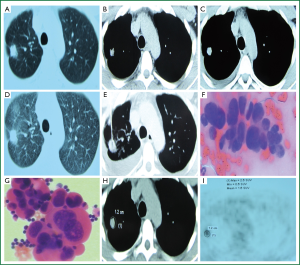
A 35-year-old man had right chest pain two months ago, which was persistent and dull, and worsened when coughing and taking deep breaths. Chest CT showed a pulmonary nodule in the upper right chest on October 5, 2013 (Figure 1A,B), which was a SPN. Anti-infection treatment with “moxifloxacin and azithromycin” was delivered for fourteen days, but no significant improvement in symptoms was observed. The patient revisited our clinic for a repeat chest CT, which showed a lesion in the upper right lung, a small amount of right pleural effusion, and multiple micro nodules in the interlobular pleura (Figure 1C,D). Chest CT suggested enlargement of the nodule and pleural effusion. The patient was otherwise healthy, with no history of exposure to radiation and toxic substances, or history of tuberculosis or smoking. Examination upon admission: body temperature 37.0 °C, pulse 65 beats/min, respiratory rate 19 breaths/min, and blood pressure 115/69 mmHg. No superficial lymph node enlargement was palpable; the neck was soft without resistance; the trachea was at the midline; the thyroid was not enlarged; enhanced breath sounds were heard at the left lung, while decreased breath sounds were noticed at the right lower part. Dullness of the right lower chest was noticed on percussion. The cardiac rhythm was regular without murmurs. The abdomen was soft and non-tender. No swelling of lower limbs was observed. The admitting diagnosis was right upper lung nodule of unknown origin with right pleural effusion. The peripheral leukocyte count upon admission was 6.0×109/L, neutrophils 0.61, ESR 5 mm/h. Total plasma protein was 72.6 g/L, albumin 48.3 g/L, lactate dehydrogenase 160 IU/L (normal reference value 1-226 IU/L). Blood carbohydrate antigen 125 (CA125) was 87.83 U/mL (normal <35.30 U/mL). All other serum tumor markers were negative, and tuberculosis antibody was negative. The CT-guided percutaneous lung biopsy showed adenocarcinoma cells (Figure 1E,F). Acid-fast smear was negative for bacilli. Chest ultrasound showed a non-echogenic area of 5.0 cm in the right chest. Closed drainage of the right chest was performed. The pleural effusion was dark yellow in appearance, and specific gravity was 1.028, WBC 1.98×109/L, neutrophil count ratio 0.25, lymphocyte ratio 0.75; albumin 47.7 g/L, lactate dehydrogenase 265 IU/L, glucose 6.36 mmol/L, adenosine deaminase 7 U/L, CEA 0.87 ng/mL, and γ-interferon 5.0 ng/L. Adenocarcinoma cells were found in the pleural effusion (Figure 1G). Abdominal ultrasound showed gallbladder polyps. No abnormal cardiac ultrasound findings were noticed. The whole body PET/CT showed a right upper lobe nodule, maximum standardized uptake value (SUVmax) 2.6, right chest effusion, small nodules in the right lung, mediastinal calcification nodules, and cholecystolithiasis (Figure 1H,I). Pleural effusion EGFR gene mutation showed wild-type exons 19, 20 and 21. Based on the patient’s clinical and pathological findings, the diagnosis was stage IV adenocarcinoma of the right upper lung with pleural metastasis. After complete drainage of the pleural effusion, the patient received pleural injection of mitomycin plus dexamethasone, in combination with a cycle of AP (pemetrexed + cisplatin) chemotherapy. He was discharged after the clinical symptoms improved.
Discussion
Since SPNs are small and associated with very few and mild clinical manifestations, particularly in early lung cancer where there is almost no symptom, clinicians often reply on various imaging methods to detect SPNs when not much clinical information is available. CT is currently considered the most sensitive imaging examination of lung nodules. In particular, the introduction of multi-slice CT has greatly improved the detection rate and qualitative accuracy in the diagnosis of pulmonary nodules. With CT technology, the differentiation of benign and malignant SPNs is mainly based on morphological characteristics (3). The basic CT morphological signs of SPNs include size, margin characteristics (lobulation, spiculation), internal structure (vacuole sign, bronchial inflation, hollow cavity sign, ground-glass density) and peripheral signs (blood vessel convergence, halo sign and pleural indentation). At initial presentation, the patient manifested right upper lung pleural indentation with glitches around on chest CT. These were suggestive of malignancy, so the patient should have received a further lung biopsy to confirm the diagnosis as soon as possible instead of anti-infection measures.
Positron emission computed tomography (PET): PET/CT combines PET that reflects the metabolic capacity of a tumor and CT that shows the organizational structure in a high-resolution way. It has a sensitivity of 97% and a specificity of 85% to SPN, and is recognized as the optimal non-invasive means of differentiating benign SPNs from malignant ones (4). According to most studies, the SUVmax of 2.5 is used as a diagnostic threshold, in which a SUVmax of ≥2.5 is suggestive of malignancy (5). Malignancies with a SUVmax of ≤2.5 are often bronchioloalveolar carcinoma and metastatic carcinoid tumors. For SPNs ≤1 cm in diameter, a SUVmax of ≥2.5 is not highly accurate in the diagnosis of malignancy.
We routinely use high-resolution chest CT scan for patients with lung lesions. In this SPN case, the signs on high-resolution CT including surrounding short burrs and “pleural indentation”; FDG imaging showed high uptakes of FDG in the lesion; conventional imaging showed a SUVmax of 2.6, and delayed imaging showed a SUVmax of 5.2, and a retention index of 100%. In addition, the patient’s CA125 was slightly increased, an antigen against lung tumor cells secreted by columnar epithelial cells and glands in the respiratory mucosa, which can be elevated in patients with lung cancer. Many studies have shown that only significantly increased CA125 levels can be statistically valuable in the diagnosis of malignant SPNs (6). Meanwhile, CEA, SCC, CYFRA21-1, NSE and other tumor markers of this patient were within the normal range. Therefore, the tumor markers could not provide further help for an accurate diagnosis.
CT-guided percutaneous fine-needle aspiration biopsy (FNAB) is an essential diagnostic technique among interventional chest radiology, one of the important methods for the diagnosis or differential diagnosis of lung diseases. With the application of CT techniques and continuous improvement of needle biopsies, there has been a substantial increase in the diagnostic accuracy of for lung diseases with CT-guided lung biopsies. A meta-analysis (7) reports that FNAB diagnosis has a sensitivity of 86% and specificity of 98.8% for malignant SPNs, while combination with CT-guided puncture will add up to a sensitivity of 91% and specificity of 94% (8). Less invasive to lung tissue, FNAB is a safe, reliable and accurate method with fewer complications. It has its unique value especially for lesions that is not accessible by bronchoscopy in the peripulmonary area. The patient underwent a CT-guided percutaneous lung biopsy, in which the needle accurately reached the lesion site and obtained specimens. No pneumothorax, hemoptysis or other complication occurred during the biopsy.
In clinical practice, for advanced lung cancer patients with sensitive EGFR gene mutations, first-line EGFR-TKI therapy is recommended; for those without sensitive EGFR gene mutations or unknown EGFR mutation status, first-line platinum-based dual drug chemotherapy is strongly recommended. This patient was positive for wild type exons 19, 20 and 21, so the AP chemotherapy was delivered, and pleural injection of chemotherapy drugs was given. The symptoms improved after the treatment.
In short, the diagnosis of SPNs is a comprehensive evaluation process. Imaging studies in combination with serum tumor markers and percutaneous lung biopsies can improve the diagnostic accuracy of SPNs. The treatment of SPNs shall take into account the nature of the nodules, individual factors and genetic findings, and require individualized treatment programs.
Acknowledgements
The study was supported by a grant from “Twelve-Five Plan”, the Major Program of Nanjing Medical Science and Technique Development Foundation (Molecular Mechanism Study on Metastasis and Clinical Efficacy Prediction of Non-small Cell Lung Cancer) and Third Level Training Program of Young Talent Project of Nanjing Health, Nanjing Medical Science and Technology Development Project (QRX11226), and Young Professionals Foundation of Nanjing Chest Hospital.
Disclosure: The authors declare no conflict of interest.
References
- Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med 2003;348:2535-42. [PubMed]
- Dargan EL. The enigma of the solitary pulmonary nodule. J Natl Med Assoc 1973;65:101-3 passim. [PubMed]
- Choromańska A, Macura KJ. Evaluation of solitary pulmonary nodule detected during computed tomography examination. Pol J Radiol 2012;77:22-34. [PubMed]
- Kim SK, Allen-Auerbach M, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 2007;48:214-20. [PubMed]
- Khalaf M, Abdel-Nabi H, Baker J, et al. Relation between nodule size and 18F-FDG-PET SUV for malignant and benign pulmonary nodules. J Hematol Oncol 2008;1:13. [PubMed]
- Bekci TT, Senol T, Maden E. The efficacy of serum carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 15-3 (CA15-3), alpha-fetoprotein (AFP) and human chorionic gonadotropin (hCG) levels in determining the malignancy of solitary pulmonary nodules. J Int Med Res 2009;37:438-45. [PubMed]
- Lacasse Y, Wong E, Guyatt GH, et al. Transthoracic needle aspiration biopsy for the diagnosis of localised pulmonary lesions: a meta-analysis. Thorax 1999;54:884-93. [PubMed]
- Cheung JY, Kim Y, Shim SS, et al. Combined fluoroscopy- and CT-guided transthoracic needle biopsy using a C-arm cone-beam CT system: comparison with fluoroscopy-guided biopsy. Korean J Radiol 2011;12:89-96. [PubMed]


