Hemodynamic assessment of atrial septal defects
Introduction
Atrial septal defects (ASD) are a group of malformations that allow shunting between the systemic and pulmonary circulation. Four types of ASDs have been described: primum, secundum, sinus venosus and coronary sinus defects. ASDs can present as isolated defects (the most common) or in association with other cardiac malformations, where they can have a significant impact on cardiovascular hemodynamics. Advances in non-invasive imaging over the last decades have changed the diagnostic evaluation of ASD. Diagnostic heart catheterization to determine anatomy and degree of ASD shunting is nowadays rarely indicated unless it is performed to assess the hemodynamic significance of associated anomalies or when pulmonary vascular obstructive disease is suspected.
Hemodynamics in isolated ASD
ASD is one of the most common congenital heart defects found in otherwise structurally normal hearts. Although the anatomy and therapeutic approach varies among the different types of ASD, pathophysiology is common to all them. ASD hemodynamics is usually described based on the secundum type, by far the most common. Hemodynamics, and consequently therapy, during childhood is usually simpler as it is limited to left-to-right shunting and right heart volume overload in otherwise normal ventricles (1). Symptoms and failure to thrive secondary to ASD in childhood are rare but well described (2,3). In adults, the hemodynamic burden of long-standing shunting becomes manifest due to progressive ventricular dysfunction and pulmonary hypertension making the therapeutic management of these patients more challenging.
An isolated ASD presents with left-to-right shunt. Regardless of the type of ASD, the direction and degree of the atrial shunting will depend on the size of the defect and the difference in diastolic compliance between the left and right ventricles. The difference in pressures between the right and left atrium is usually minimal and certainly does not explain shunting. Left-to-right shunting occurs during late ventricular systole and early diastole, increasing during atrial contraction and expiration. ASDs associated with normal size of right heart structures are usually considered of no hemodynamic significance. Defects with shunts >1.5:1 are considered significant and are responsible for different degrees of right heart volume overload. Right ventricular dilation and diastolic septal shift towards the left compromise the diastolic filling of the left ventricle. As a result, left-to-right shunting at the atrial level increases even further potentially compromising systemic cardiac output (4). Changes of the ventricular geometry modify normal biventricular interaction and eventually affect ventricular function. Left ventricular systolic dysfunction has been described in patients with large ASDs (5). Physiologic changes in ventricular compliance that occur with age modify the degree of shunting as well. Most patients with large ASDs become symptomatic by the third or fourth decade of life as aging decreases left ventricular compliance and augments the left-to-right shunt. Late clinical sequela associated with long-standing ASD and right heart volume overload include atrial arrhythmias, exercise intolerance, dyspnea, fatigue, paradoxical embolization and pulmonary hypertension. Life expectancy is reduced in untreated ASD (6,7). Pulmonary artery pressure (PAP) is usually normal in children with ASD but can be mildly increased in young patients with large defects. Chronic volume overload can trigger remodeling of the pulmonary vascular bed with myointimal cell proliferation, increased medial smooth muscle and increased collagen leading to arteriolar narrowing and pulmonary hypertension. Progressive pulmonary vascular disease and severe pulmonary artery hypertension develop in less than 1% of patients, predominantly in females (8). However, its etiology is likely multifactorial, and not only dependent on shunt degree and duration. Eventually, pulmonary pressures can reach systemic levels with shunt reversal at the atrial level, i.e., Eisenmenger syndrome. Development of pulmonary artery hypertension later in life can occur following ASD closure at an earlier age (9-11).
For the usual patient with left-to-right shunting due to an isolated ASD, cardiac catheterization is not required (12,13). Hemodynamic evaluation is indicated in patients with abnormal systemic saturation which suggests right-to-left shunting at the defect, or when elevated PAP is suspected on echocardiography. Transthoracic echocardiography is the primary diagnostic method to assess ASD including location, size and hemodynamics. The most pronounced echocardiographic finding associated with a left-to-right shunt is dilation of the right ventricle, which can be assessed with different echocardiographic methods. Doppler peak systolic tricuspid regurgitation pressure gradient is a reliable noninvasive method for the evaluation of pulmonary artery systolic pressure. Mean and diastolic PAPs can be estimated from pulmonary valve regurgitation Doppler signal. Bidirectional shunting across the ASD on Doppler interrogation, right ventricular hypertrophy and systolic flattening of the interventricular septum are also indicators of elevated pulmonary pressures. Echocardiography is also used during transcatheter intervention of ASD. Device closure of secundum ASD is routinely performed in the catheterization laboratory (14) but anecdotal case reports of transcatheter closure of sinus venosus and coronary sinus defects have been published (15,16).
Angiographic evaluation of ASDs yields in most cases unsatisfactory imaging, thus the significance of an atrial communication is determined based on the changes of oxygen saturation through the cardiac chambers. An increase in oxygen saturation of 10% or more at the atrial level from the superior vena cava (SVC) and the persistence of the high saturation in the right ventricle and pulmonary arteries indicate the presence of left-to-right shunting at the atrial level. Reproducibility increases the sensitivity and a 5% step up on oxygen saturation is considered significant if it can be reproduced in two or more series. The SVC saturation is used because it has the most predictable systemic venous saturation and is usually similar to the pulmonary artery saturation in the absence of shunting. The inferior vena cava has a wide oxygen saturation variability due to the streaming from the highly oxygenated renal veins and the low oxygen levels from the lower parts of the body and is therefore unreliable. The low oxygenated blood from the coronary sinus (except in coronary sinus defects) also drains into the right atrium and can confound oximetry results. Because different venous systems drain into the right atrium, blood samples should consistently be obtained in the same area of the chamber, with the catheter facing the lateral atrial wall. A pulmonary-to-systemic flow ratio ≥1.5:1 is considered significant and should prompt closure of the defect. Operators need to be aware of conditions that may alter the SVC saturation such as significant tricuspid regurgitation (particularly in the presence of a ventricular septal defect) or anomalous pulmonary venous return which may yield a falsely elevated SVC saturation and mask the presence of an atrial communication. Interestingly, due to increased pulmonary flow, a gradient can occasionally be measured across the pulmonary veins in the presence of significant left-to-right shunting. In patients with borderline systemic saturation and unclear shunting at the atrial level, hemodynamic assessment should include oxygen saturation samples from the pulmonary veins to rule out other sources of systemic desaturation (17,18).
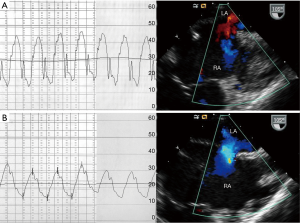
Pulmonary hypertension is defined as a mean PAP of more than 25 mmHg at rest or more than 30 mmHg during exercise (19). Vasoreactivity testing with FiO2 100% and/or nitric oxide can be performed to assess pulmonary vascular reactivity (Figure 1), ASD closure is contraindicated in patients with irreversible pulmonary hypertension and no evidence of left-to-right shunting (20). Provided that pulmonary vascular disease has not progressed irreversibly, closure of any hemodynamically significant defect is recommended regardless of age even in asymptomatic patients. However, the risk of atrial arrhythmias is not reduced by closure of the defect. In fact, age and pulmonary pressure are predictive factors of atrial arrhythmias, both before and after ASD closure (21). Normalization of PAP after defect closure occurs less frequently with higher pressures at the time of diagnosis and in older patients (22).
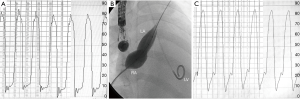
Partial occlusion of ASD with a fenestrated surgical patch/device can be beneficial in patients with borderline hemodynamics in whom complete closure is deemed unsafe. Individuals with impaired left ventricular diastolic function may manifest abrupt rise in left atrial and left ventricular filling pressures once the ASD is completely occluded, which may result in pulmonary edema, pulmonary hypertension and atrial or ventricular arrhythmias. This phenomenon has been mainly described in older patients with diastolic dysfunction undergoing transcatheter closure of secundum ASD (23-25). Transient balloon occlusion of the ASD with simultaneous pulmonary capillary wedge/left atrial or left ventricular pressure measurement is recommended in older patients particularly in the presence of left ventricular dysfunction. An increase on left atrial or pulmonary capillary wedge pressure to ≥20 mmHg or by more than 10 mmHg from the baseline value during test balloon occlusion could lead to the development of pulmonary edema after device closure, thus the procedure should be abandoned (Figure 2). Preconditioning, using oral diuretics, ACE inhibitor or an angiotensin receptor blocker, has been shown to reduce left atrial pressure to acceptable levels in most patients with a significant left atrial pressure increase during balloon occlusion (5,26,27). Hemodynamic assessment and management of patients with ASD in the presence of pulmonary hypertension +/− right ventricular dysfunction is challenging. Successful closure of ASD following aggressive treatment with pulmonary vasodilators has been reported. Fenestrated closure of the defect to allow decompression of the right atrium may be an alternative in patients with more advanced disease (23,28-30).
Hemodynamics in ASD associated with other congenital heart defects
ASDs are commonly found in association with a wide spectrum of congenital heart conditions. As in isolated ASD, the anatomy and hemodynamic burden associated with the defect is usually determined by echocardiography alone. Need of diagnostic catheterizations to assess ASD hemodynamics during the neonatal period is rare. Most procedures performed during this period aim at increasing the size of restrictive atrial communications via balloon atrial septostomy. Diagnostic procedures later in life are usually performed to determine the need for closure of the defect and/or suitability for single or two-ventricle repair.
Although ASD can be a part of a wide spectrum of anomalies, cardiac malformations in which the presence of ASD alters hemodynamics can usually be included in one of the following categories:
Dextro-transposition of the great arteries (D-TGA)
In D-TGA, the systemic and pulmonary circulations run in parallel, rather than in series, thus this lesion is not compatible with survival without adequate mixing between both circulations. Mixing can occur at three levels: across atrial and ventricular communications and at the ductus arteriosus. In patients with no ventricular septal defect and a restrictive atrial communication, mixing occurs mainly at the ductus arteriosus which, even with prostaglandin therapy, may not ensure proper systemic oxygenation resulting in acidosis and eventually circulatory collapse. When the ductus is the main/only source of mixing, oxygen saturation tends to be lower in the upper extremities and higher in the lower extremities, the so-called transposition physiology (31). The presence of arterial hypoxemia and transposition physiology suggest lack of enough mixing at the atrial level and prompts the need for balloon atrial septostomy. Newborns with a ventricular septal defect can also require a balloon atrial septostomy, but it is less common.
Small left sided structures
This group encompasses a wide spectrum of conditions with different degrees of left ventricular hypoplasia, which compromises antegrade flow through the left heart and eventually systemic cardiac output. Therefore, there is an obligatory left-to-right shunt across the ASD. The hemodynamic role of the ASD will vary depending on the underlying condition. A restrictive communication can be life threatening as it occurs in hypoplastic left heart syndrome (HLHS) with restrictive atrial communication. A wide-open communication is essential in individuals committed to single-ventricle palliation. On the other hand, some degree of obstruction may be beneficial in patients who can eventually achieve a biventricular circulation, in order to force more flow across the mitral valve.
Newborns with hypoplastic left heart structures, including a small left ventricle, aortic outflow tract obstruction, small mitral valve, and aortic arch hypoplasia undergo single ventricle palliation and require an unrestrictive atrial communication. As part of the Norwood procedure, an atrial septectomy is routinely performed. In the subset of HLHS with a restrictive atrial septum, patients present soon after birth with severe hemodynamic instability due to impeded egress of left atrial flow, which results in a marked increase of pulmonary venous pressure, hypoxemia and acidosis. Indications for creation or enlargement of an existing communication include a variable combination of progressive hypoxemia, respiratory distress, acidosis, chest-X-ray “whiteout” and elevated Doppler mean gradient across the atrial communication (>9–10 mmHg) (32). A similar situation occurs in HLHS status-post hybrid palliation, where the ASD becomes restrictive over time often requiring intervention to relieve the left atrial hypertension. Indications for intervention in this group include a mean atrial septal gradient of ≥6 mmHg, feeding difficulties or a systemic saturation <80% (33).
However, patients with borderline small left heart structures may be candidates for biventricular circulation (34,35). Recent experience with fetal aortic valvuloplasty has increased the number of newborns with borderline left heart structures who otherwise would have undergone single ventricle palliation (36). Growth of left-sided structures can be promoted by a variety of procedures in these patients. ASD is a key component in the hemodynamic management of patients being considered for biventricular circulation. Some may not tolerate total occlusion of the ASD due to a significant rise in left atrial pressures. On the other hand, a mild/moderate degree of ASD restriction is beneficial as it has been associated with improved growth of left heart dimensions and eventual biventricular circulation. Patients with an intact ventricular septum who have a small ASD can be evaluated by cardiac catheterization and balloon occlusion of the ASD to assess left atrial pressure while the left ventricle is handling a full cardiac output. Although there is not a threshold pressure beyond which biventricular circulation is contraindicated, a left atrial pressure less than 20 mmHg during balloon occlusion has generally been considered acceptable. In patients undergoing fenestrated closure, the ASD can be closed with a patch with a 4 mm fenestration. This provides a balance between decompression of the left atrium and maintenance of adequate cardiac output (34,35).
Pulmonary/tricuspid valve hypoplasia with hypoplastic right ventricle
Patients with these conditions present with different degrees of cyanosis due to obligatory right-to-left shunting across the ASD as the right heart structures are unable to fully accommodate antegrade flow. In tricuspid atresia, the entire systemic venous return crosses the atrial septum, thus a small atrial communication may result in hemodynamic compromise. This finding is uncommon during the neonatal period but can develop later in life. Patients with a small ASD present with elevated right atrial pressure, liver congestion and eventually low cardiac output. A heart catheterization is indicated to confirm the diagnosis and eventually enlarge the defect with balloon septostomy, static balloon angioplasty, or stent placement.
In certain conditions, right-to-left shunting across the atrial defect can spontaneously decrease over time, resulting in progressive increase of the systemic saturation. Newborns with severe pulmonary valve stenosis can present with cyanosis secondary to right ventricular hypertrophy and poor diastolic compliance. Following balloon valvuloplasty, systemic saturation is expected to increase over the following weeks as hypertrophy regresses and ventricular compliance improves. Likewise, patients with Ebstein’s disease can present with profound cyanosis at birth due to tricuspid regurgitation and abnormal ventricle compliance. As pulmonary vascular resistance drops, tricuspid regurgitation improves decreasing the degree of right-to-left shunt at the atrial level (4).
Pulmonary atresia/intact ventricular septum is often associated with some degree of tricuspid valve hypoplasia and poor right ventricular compliance causing right-to-left shunting across the ASD. First stage palliation in these patients includes surgical or transcatheter opening of the right ventricular outflow tract and a Blalock Taussig shunt or stenting of the ductus arteriosus. A catheterization is usually performed at a later follow up to assess suitability for ASD and shunt closure. Patients with significantly hypoplastic tricuspid valve or elevated right ventricular diastolic pressure may not tolerate ASD closure. In order to rule out tricuspid valve stenosis, temporary balloon occlusion of the ASD is performed with simultaneous measurement of the right atrial pressure. A rise on the right atrial pressure suggests the presence of either tricuspid valve stenosis or a non-compliant right ventricle which can be differentiated by simultaneous measurement of the right atrial and ventricular pressures. Changes on the diastolic tricuspid valve flow can also be assessed by Doppler interrogation during ASD balloon occlusion. Patients with hypoplastic tricuspid valves unable to handle full cardiac output should be considered for a bidirectional cavopulmonary anastomosis and ASD closure, i.e., 1 ½ ventricle repair. In those in whom right atrial and systemic pressure remain stable and arterial blood is fully saturated during balloon occlusion, the ASD can be safely closed (18).
Complex congenital heart disease
Newborns with total anomalous pulmonary venous return to the systemic venous circulation or equivalent hemodynamic conditions need a large atrial communication in order to maintain systemic cardiac output. The diagnosis and need of balloon septostomy if the ASD is restrictive is usually made by echocardiography.
Single-ventricle patients: the Fontan operation is the last surgical stage in patients undergoing single-ventricle palliation. Fenestration of the Fontan pathway has traditionally been used to decrease surgical morbidity and mortality, particularly in high-risk patients (37). Transcatheter closure is considered in patients in whom fenestration remains patent at follow up. Indications for elective closure of a fenestration remains controversial, particularly in the absence of symptoms. Patients with a resting arterial oxygen saturation ~85% or those who develop significant desaturation with exercise should be considered for fenestration closure, if transient balloon occlusion during catheterization is tolerated. Careful consideration should be given to patients with elevated Fontan pathway pressures at baseline and those in whom temporary balloon occlusion results in an increase in venous pressure >16 mmHg or a cardiac index drop to less than 2 L/m/m2 as fenestration closure may not be tolerated in the long term (38,39).
Conclusions
Hemodynamics in children with isolated ASD is usually straight forward and require no further evaluation unless there is clinical or echocardiographic evidence of elevated PAP. Adult patients diagnosed with ASD should be carefully evaluated for the burden associated with long-standing shunting such as ventricular dysfunction and pulmonary artery hypertension. ASD closure should be attempted regardless of age in all patients with acceptable hemodynamics. Long-term follow up after ASD closure is recommended as pulmonary hypertension can develop later in life. Evaluation of ASD in the presence of other congenital heart defects should be individualized as it will vary depending on the baseline anatomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Allen HD, Shaddy RE, Penny DJ, et al. Moss and Adams' heart disease in infants, children, and adolescents: including the fetus and young adult. 9th ed. Philadelpha: Lippincott Williams & Wilkins, 2016.
- Lammers A, Hager A, Eicken A, et al. Need for closure of secundum atrial septal defect in infancy J Thorac Cardiovasc Surg 2005;129:1353-7. [Crossref] [PubMed]
- Bishnoi RN, Everett AD, Ringel RE, et al. Device closure of secundum atrial septal defects in infants weighing less than 8 kg. Pediatr Cardiol 2014;35:1124-31. [Crossref] [PubMed]
- Garson A, Bricker JT, Fisher DJ, Neish SR. The Science and Practice of Pediatric Cardiology. 2nd ed. Maryland: Williams & Wilkins, 1998.
- Masutani S, Senzaki H. Left ventricular function in adult patients with atrial septal defect: implication for development of heart failure after transcatheter closure. J Card Fail 2011;17:957-63. [Crossref] [PubMed]
- Campbell M, Neill C, Suzman S. The prognosis of atrial septal defect. Br Med J 1957;1:1375-83. [Crossref] [PubMed]
- Diaconu CC. Atrial septal defect in an elderly woman-a case report. J Med Life 2011;4:91-93. [PubMed]
- Besterman E. Atrial septal defect with pulmonary hypertension. Br Heart J 1961;23:587-98. [Crossref] [PubMed]
- van Riel AC, Schuuring MJ, van Hessen ID. Contemporary prevalence of pulmonary arterial hypertension in adult congenital heart disease following the updated clinical classification. Int J Cardiol 2014;174:299-305. [Crossref] [PubMed]
- Engelfriet PM, Duffels MGJ, Moller T. Pulmonary arterial hypertension in adults born with a heart septal defect: The Euro Heart Survey on adult congenital heart disease. Heart 2007;93:682-7. [Crossref] [PubMed]
- Lau EM, Manes A, Celermajer DS, et al. Early detection of pulmonary vascular disease in pulmonary arterial hypertension: time to move forward Eur Heart J 2011;32:2489-98. [Crossref] [PubMed]
- Freed MD, Nadas AS, Norwood WI, et al. Is routine preoperative cardiac catheterization necessary before repair of secundum and sinus venosus atrial septal defects? J Am Coll Cardiol 1984;4:333-6. [Crossref] [PubMed]
- Shub C, Tajik AJ, Seward JB, et al. Surgical repair of uncomplicated atrial septal defect without "routine" preoperative cardiac catheterization. J Am Coll Cardiol 1985;6:49-54. [Crossref] [PubMed]
- Kazmouz S, Kenny D, Cao QL, et al. Transcatheter closure of secundum atrial septal defects. J Invasive Cardiol 2013;25:257-64. [PubMed]
- Torres A, Gersony WM, Hellenbrand W. Closure of unroofed coronary sinus with a covered stent in a symptomatic infant. Catheter Cardiovasc Interv 2007;70:745-8. [Crossref] [PubMed]
- Crystal MA, Vincent JA, Gray WA. The wedding cake solution: A percutaneous correction of a form fruste superior sinus venosus atrial septal defect. Catheter Cardiovasc Interv 2015;86:1204-10. [Crossref] [PubMed]
- Braunwald E, Bonow RO. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia: Elsevier Saunders, 2012.
- Lock JE, Kean JF, Perry SB. Diagnostic and Interventional Catheterization in Congenital Heart Disease. 2nd ed. Massachusetts: Kluwer Academic Publishers, 2001.
- Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004;351:1655-65. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults With Congenital Heart Disease. Circulation 2008;118:e714-833. [Crossref] [PubMed]
- Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med 1999;340:839-46. [Crossref] [PubMed]
- Gabriels C, De Meester P, Pasquet A, et al. A different view on predictors of pulmonary hypertension in secundum atrial septal defect. Int J Cardiol 2014;176:833-40. [Crossref] [PubMed]
- Abdelkarim A, Levi DS, Tran B, et al. Fenestrated Transcatheter ASD Closure in Adults with Diastolic Dysfunction and/or Pulmonary Hypertension: Case Series and Review of the Literature. Congenit Heart Dis 2016;11:663-71. [Crossref] [PubMed]
- Rich S, Dodin E, McLaughlin VV. Usefulness of atrial septostomy as a treatment for primary pulmonary hypertension and guidelines for its application. Am J Cardiol 1997;80:369-71. [Crossref] [PubMed]
- Holzer R, Cao QL, Hijazi ZM. Closure of a moderately large atria l septal defect with a self-fabricated fenestrated Amplatzer septal occlude in an 85-year-old patient with reduced diastolic elasticity of the left ventricle. Catheter Cardiovasc Interv 2005;64:513-8. [Crossref] [PubMed]
- Schubert S, Peters B, Abdul-Khaliq H, et al. Left ventricular conditioning in the elderly patient to prevent congestive heart failure after transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv 2005;64:333-7. [Crossref] [PubMed]
- Gruner C, Akkaya E, Kretschmar O, et al. Pharmacologic preconditioning therapy prior to atrial septal defect closure in patients at high risk for acute pulmonary edema. J Interv Cardiol 2012;25:505-12. [Crossref] [PubMed]
- de Lezo JS, Medina A, Romero M, et al. Effectiveness of percutaneous device occlusion for atrial septal defect in adult patients with pulmonary hypertension. Am Heart J. 2002;144:877-80. [Crossref] [PubMed]
- Gonzalez-Barlatay F, Fournier A, Raboisson MJ, et al. Atrial Septal Defect Closure with Occlutech. ASD Fenestrated Device in a Child with Severe Pulmonary Hypertension. Pediatr Cardiol 2017;38:202-5. [Crossref] [PubMed]
- Hirsch R, Bagby MC, Zussman ME. Fenestrated ASD closure in a child with idiopathic pulmonary hypertension and exercise desaturation. Congenit Heart Dis 2011;6:162-6. [Crossref] [PubMed]
- Villafañe J, Lantin-Hermoso MR, Bhatt AB, et al. D-transposition of the great arteries: the current era of the arterial switch operation. J Am Coll Cardiol. 2014;64:498-511. [Crossref] [PubMed]
- Hoque T, Richmond M, Vincent JA, et al. Current outcomes of hypoplastic left heart syndrome with restrictive atrial septum: a single-center experience. Pediatr Cardiol 2013;34:1181-9. [Crossref] [PubMed]
- Holzer RJ, Wood A, Chisolm JL, et al. Atrial septal interventions in patients with hypoplastic left heart syndrome. Catheter Cardiovasc Interv 2008;72:696-704. [Crossref] [PubMed]
- Kalish BT, Banka P, Lafranchi T, et al. Biventricular conversion after single ventricle palliation in patients with small left heart structures: short-term outcomes. Ann Thorac Surg 2013;96:1406-12. [Crossref] [PubMed]
- Emani SM, McElhinney DB, Tworetzky W, et al. Staged left ventricular recruitment after single-ventricle palliation in patients with borderline left heart hypoplasia. J Am Coll Cardiol 2012;60:1966-74. [Crossref] [PubMed]
- Freud LR, McElhinney DB, Marshall AC, et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation 2014;130:638-45. [Crossref] [PubMed]
- Bridges ND. Fenestration of the Fontan baffle: Benefits and complications. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 1998;1:9-14. [Crossref] [PubMed]
- Kreutzer J, Graziano JN, Stapleton G, et al. Late catheter interventions in hypoplastic left heart syndrome. Cardiol Young 2011;21 Suppl 2:65-76. [Crossref] [PubMed]
- Cowley CG, Badran S, Gaffney D, et al. Transcatheter closure of Fontan fenestrations using the Amplatzer septal occluder: initial experience and follow-up. Catheter Cardiovasc Interv 2000;51:301-4. [Crossref] [PubMed]


