Extending the curve: survival of EGFR-mutated lung cancer patients in the 21st century
Lung cancer remains the most lethal malignancy worldwide (1,2). Although incidence rates have decreased in part of the developed world, other regions face the challenge of an epidemic of paramount importance, which is likely to take hundreds of thousands of lives in the coming years (2,3).
In the last two decades the world has undeniably produced some of the greatest advances in lung cancer therapy. Enormous breakthroughs have been achieved through the discovery of actionable mutations and immune checkpoints which have given rise to targeted therapies and immune checkpoint inhibitors, improving the outlook for subgroups of advanced lung cancer patients worldwide (3,4). This is of particular relevance, since the second half of the 20th century was characterized by an unchallenged status quo for lung cancer treatment, which included the use, alone or in combination, of surgery, radiotherapy and chemotherapy (3). The scarce therapeutic options were further limited by their lack of long-term efficacy, with a 5-year survival rate for lung cancer patients which ranged from 5–16%, and which remained unchanged from the 1970’s to the dawn of this new century (3).
By the early years in the 21st century the promise of targeted therapy was beginning to loom over with several compounds showing astounding responses in preclinical and early clinical studies. The novel therapeutic approaches, along with a refined vision of the integral care of lung cancer patients has produced a new outlook regarding the future therapeutic approach to this malignant disease. Agents such as erlotinib, afatinib and gefitinib, FDA-approved for lung cancer treatment in 2004 (5), 2013 (6), and 2015 (7) respectively, have more than doubled progression-free survival (PFS) for patients with non-small cell lung cancer (NSCLC) with actionable epidermal growth factor receptor (EGFR) mutations, in addition to improving objective response rates (ORR), duration of response, quality of life and decreasing treatment-related toxicity. This highly personalized approach, which usually makes use of molecular biology techniques, next-generation sequencing and targeted therapies is commonly referred to as precision medicine, and is now considered the standard of care in NSCLC therapy, particularly for patients with adenocarcinoma histology (8).
Last November a clinical trial was published by the FLAURA investigators, and results from this study have shifted the paradigm once again (9). In this phase 3 study performed by Soria et al., 556 patients with untreated, EGFR-mutated advanced NSCLC were randomized to receive osimertinib (80 mg once a day) or a standard TKI (gefitinib 250 mg daily; erlotinib 150 mg daily). Osimertinib represents the third generation of EGFR-tyrosine kinase inhibitors (EGFR-TKIs), and presents several advantages compared to previous-generation compounds such as erlotinib, gefitinib and afatinib (10). In vitro studies have shown that third-generation compounds are much more potent compared to their older counterparts, with high efficacy against the mutation-type spectrum of EGFR(+) lung cancer and with striking mutation specificity (11). Other upgrades in regards specifically to osimertinib include its ability to cross the blood-brain barrier and the selective inhibition of classic EGFR resistance mutation T790M, making it a feasible therapeutic option even after the acquisition of this resistance mechanism. Since it received accelerated approval by the FDA in November 2015, further studies using osimertinib continue to add to the body of evidence which supports its use in the second, and now, first-line setting (11).
In this recently published clinical trial Soria et al., report an impressive 18.9 PFS for patients treated with osimertinib, compared to 10.2 months with standard TKIs, an ORR of 80% and an unparalleled 18-month survival rate of 83%. In addition to the therapeutic efficacy, osimertinib also produced fewer grade 3 or higher adverse events compared to standard TKIs (34% vs. 45%), highlighting the molecule’s ability to inhibit mutated EGFR while sparing the physiological wild-type molecule, which translates to a better tolerability profile, with fewer adverse events (9,10).
The dramatic change in the therapeutic pipeline for EGFR-mutated NSCLC which has been inherently proposed by the results from this trial cannot be denied. The ramifications of these observations include the fact that this will raise the bar for research currently focused on improving TKIs and compounds which are already in advanced research phases. Such is the case of dacomitinib, a second-generation TKI which showed promise in previous studies, but which is likely to lose momentum if its efficacy profile does not measure up to the new standard imposed by osimertinib (12). Likewise, first and second generation-TKIs will start to become outdated as these new compounds prove their efficacy in the first-line setting. This must be carefully considered, as it will likely create a bottleneck of available treatments for the second, and further, line setting and it is still unknown if first and second generation TKIs will be useful in this line.
On the other hand, clinical trials for osimertinib (Table 1) have focused on patients with common EGFR mutations (exon 19 deletions and L858R), meanwhile, patients with non-common EGFR mutations, continue to be underrepresented, and targeted therapeutic options for them are limited to first-line afatinib (13). It would then be highly relevant to include these patient subgroups in future clinical trials. Another question which might be relevant is the potential role of liquid biopsies in patients receiving osimertinib therapy, in order to timely identify mutations such as C797S, which confers resistance to osimertinib, although it might restore sensitivity to first-generation TKIs (14). In the same way, what to do with the appearance of other putative resistance mechanisms after the initial use of osimertinib, including amplification of MET, EGFR and KRAS, MEK1, KRAS, JAK2 or PIK3CA mutations, and HER2 exon 20 insertions. It's just a matter of time before a surge of information becomes available, for example studies like the APPLE (EORTC 1613) are attempting to understand if a sequenced strategy (gefitinib followed by osimertinib) is of value compared with upfront osimertinib regarding clinical efficacy and the occurrence of brain metastases, and at the same time, explores whether liquid biopsies could become the new standard procedure for defining disease progression versus classical RECIST criteria (15).

Full table
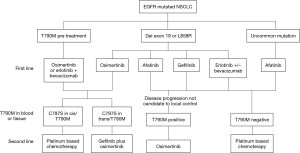
Last, it is very important to address the issue of accessibility for these novel therapeutic agents. Treatment with targeted therapy has undeniably increased the cost of treating cancer patients worldwide, and while their cost-effectiveness has been well documented, the financial burden which will be imposed in the following years will strain health systems and insurance payers. It was estimated that between 2005 and 2010, 74% of the increase in the cancer drugs expenditures was due to an increase in the use of targeted therapy agents (16), and it is likely that this will continue to rise as more targeted options become available in the first-line setting and as combinations begin to be used. It is therefore so important, perhaps now more than ever, that we find new strategies to globally tackle the issue of treatment inequity. It is known that the developing world accounts for merely 5% of the global resources devoted to cancer, despite showing the most alarming increases in lung cancer incidence and mortality (17). The question now facing most clinicians is how will we be able to give access to these therapies? Will we now through this innovation in precision medicine be opening a breach between patients depending on their economic status? Will dying from cancer become an issue for low-income nations? Hopefully government authorities will be able to give answers and propose strategies in order to close the gap which seems to be widening for lung cancer treatment worldwide. For clinicians and researchers, it is a new era full of options (Figure 1), doubts, and long survival curves, those that represent the birth of a chronic disease.
Acknowledgements
The authors would like to acknowledge all participating members of the CLICaP (Latin-American Consortium for the Investigation of Lung Cancer) for their enthusiastic labor advancing lung cancer research.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Arrieta O, Guzman-de Alba E, Alba-Lopez LF, et al. National consensus of diagnosis and treatment of non-small cell lung cancer. Rev Invest Clin 2013;65 Suppl 1:S5-84. [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med 2005;172:523-9. [Crossref] [PubMed]
- Barron F, de la Torre-Vallejo M, Luna-Palencia RL, et al. The safety of afatinib for the treatment of non-small cell lung cancer. Expert Opin Drug Saf 2016;15:1563-72. [Crossref] [PubMed]
- FDA. Erlotinib LABEL. Available onine: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021743s14s16lbl.pdf
- Institute NC. FDA Approval for Afatinib Dimaleate. Available onine: https://www.cancer.gov/about-cancer/treatment/drugs/fda-afatinibdimaleate
- FDA. Gefitinib LABEL. Available onine: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206995s000lbl.pdf
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372:793-5. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Greig SL. Osimertinib: First Global Approval. Drugs 2016;76:263-73. [Crossref] [PubMed]
- Hirano T, Yasuda H, Tani T, et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget 2015;6:38789-803. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Kanthala S, Pallerla S, Jois S. Current and future targeted therapies for non-small-cell lung cancers with aberrant EGF receptors. Future Oncol 2015;11:865-78. [Crossref] [PubMed]
- Yu HA, Tian SK, Drilon AE, et al. Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase Domain. JAMA Oncol 2015;1:982-4. [Crossref] [PubMed]
- Remon J, Menis J, Hasan B, et al. The APPLE Trial: Feasibility and Activity of AZD9291 (Osimertinib) Treatment on Positive PLasma T790M in EGFR-mutant NSCLC Patients. EORTC 1613. Clin Lung Cancer 2017;18:583-8. [Crossref] [PubMed]
- Shih YC, Smieliauskas F, Geynisman DM, et al. Trends in the Cost and Use of Targeted Cancer Therapies for the Privately Insured Nonelderly: 2001 to 2011. J Clin Oncol 2015;33:2190-6. [Crossref] [PubMed]
- CanTreat I. Scaling up cancer diagnosis and treatment in developing countries: what can we learn from the HIV/AIDS epidemic? Ann Oncol 2010;21:680-2. [Crossref] [PubMed]


