Successful extracorporeal cardiopulmonary resuscitation in a postpartum patient with amniotic fluid embolism
Introduction
Amniotic fluid embolism (AFE) is thought to occur as the result of a maternal abnormal immune response to fetal material entering the pulmonary circulation (1). AFE is a rare disease, but it is a potentially fatal condition as well as one of the leading causes of unpredictable maternal death in developed countries (2). The mortality rate reported by case studies varies from 20% to 60%, and has become reduced gradually; however, the outcomes in cardiac arrest patients are still poor (3,4). The main treatment of AFE is supportive care and the benefit of mechanical circulatory support is still controversial. Herein, we report our recent case of extracorporeal cardiopulmonary resuscitation (ECPR) for postpartum cardiac arrest with AFE and severe disseminated intravascular coagulation (DIC). In addition, we describe classic findings of AFE from an autopsy case.
Case presentation
A 32-year-old woman at 39.1 weeks of gestation was admitted to the hospital for an induction delivery. The patient had had one previous uncomplicated pregnancy with spontaneous vaginal delivery. The patient had been performing regular prenatal care, and her routine prenatal screening tests were normal. She had normal vital signs and had no history of any allergies. After 4 hours of labor induction with oxytocin, an analgesic epidural catheter was inserted into the lumbar spine, and then ropivacaine (7.5 mg/mL) and fentanyl (2 μg/mL) were continuously administered (4 mL/h). The cervix was entirely opened after 5 hours and 40 minutes of induction. At that time of changing to delivery position, the patient presented with a generalized tonic-clonic seizure and her blood pressure fell to 65/45 mmHg. Simultaneously, a neonate was delivered; the 1- and 5-minute Apgar scores were 7 and 9, respectively, and pH of blood in the umbilical cord stump was 7.270. Four mg of intravenous Lorazepam was administered immediately. Her femoral pulse was very weak and she developed apnea. We immediately performed cardiopulmonary resuscitation and endotracheal intubation. Although the blood pressure and electrical rhythm were initially restored, ventricular tachycardia occurred and then it repeatedly eliminated the arterial pulse. Bedside transthoracic echocardiography revealed a global hypokinesia of the left ventricle with severe systolic dysfunction. Laboratory data showed a profound DIC with and unmeasurable prothrombin time, an activated partial thromboplastin time, and fibrinogen level (Figure 1). Carbetocin as an uterotonic agent administration was performed to control vaginal bleeding. The amount of the vaginal bleeding was approximately 400 mL and was decreasing. A trans-vaginal ultrasound revealed no uterine atony, laceration or placental remnant.
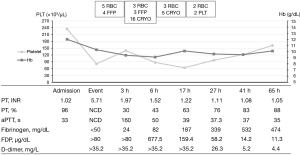
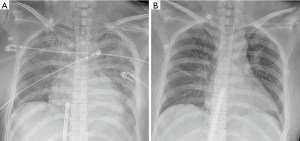
Although profound DIC was present, extracorporeal life support was utilized, considering the marginal hemodynamic status and the severe cardiac dysfunction which potentially resulted from AFE. At 1 hour after the first cardiac arrest, a 17-Fr arterial and 21-Fr venous catheter were inserted into the left femoral vessels through the Seldinger technique. We performed ECPR using the Rotaflow Centrifugal Pump® (MAQUET Cardiopulmonary AG, Hirrlingen, Germany) without any systemic anticoagulation such as bolus or continuous injection of heparin. The initial flow rate was 3.9 L/min (90% of total circulation) and a mean arterial pressure was 80 mmHg with a very narrow pulse pressure, which indicated a loss of cardiac contraction. Most of the vasoactive agents could be discontinued, and the vital signs of the patient became stable gradually. The flow of extracorporeal membrane oxygenation (ECMO) could be reduced to 2.7 L/min (60% of total circulation), and the patient regained her consciousness and orientations 2 hours after ECMO support. A follow-up echocardiography after 20 hours of ECMO support showed a marked improvement in the left ventricular systolic function. After 24 hours of ECMO support, the diffuse pulmonary edema was improved (Figure 2) and successful weaning from ECMO could be performed. Then the patient was extubated the next day. The patient received 11 units of red-blood-cell concentrates (200 mL/unit), 7 units of fresh frozen plasma (200 mL/unit), 2 units of apheresis platelets (250 mL/unit), and 21 units of cryoprecipitate (40 mL/unit) during the ECMO support (Figure 1). The patient was discharged without any neurological sequelae and cardiac complications after 13 days of hospitalization.
Discussion
Maternal cardiac arrest during childbirth is a rare event, occurring in approximately 1 in 12,000 deliveries according to US data. Except for bleeding, AFE is one of the most common causes of peripartum cardiac arrest, and the mortality rate is quite high, approximately 50% (4). Risk factors for AFE include advanced maternal age, cesarean delivery, placenta previa or abruption, and multiple pregnancies (5). In addition, as with this patient, medical induction of labor seems to increase the risk of AFE (6). But, AFE with or without these risk factors, cannot be predicted or prevented. Therefore, AFE should be considered in the differential diagnosis of a sudden cardiopulmonary collapse of any pregnant or recent postpartum patient.
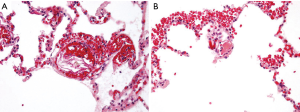
Diagnosis of AFE is based on the clinical situations including the classical triad of respiratory distress, cardiovascular collapse, and coagulopathy. Pathophysiology of AFE remains incompletely understood, but the complex sequence of hemodynamic alterations triggered in peripartum women by entrance of fetal material into the maternal circulation, results in abnormal activation of proinflammatory mediator systems and mechanical obstruction (1,7). For reference, we could describe the typical pathologic features of an autopsy case of a 29-year-old postpartum woman (Figure 3). As a result, pulmonary vasoconstriction and hypertension progress to the acute right ventricular failure was followed by left ventricular failure, and coagulation cascade was activated. But, the initial pulmonary hypertension may be transient, as severe left ventricular dysfunction with normal pulmonary arterial pressures appear to be dominant hemodynamic changes in some patients (7,8). Various degrees of DIC are present in up to 80% of the cases (9) and these are commonly manifested by hemorrhagic complications.
If cardiorespiratory collapse with AFE occurred before childbirth, prompt delivery is important for both maternal and neonatal prognosis, and this feat is accomplished with a decreased risk of permanent neurological damage from anoxia (7). If cardiac output has not yet been effectively established, relieving aortocaval compression by emptying the uterus significantly improves resuscitative efforts. The ECPR may be considered when conventional cardiopulmonary resuscitation proves to be refractory (10). However, in this situation, the use of an anticoagulation during the ECMO is a common clinical dilemma because bleeding may worsen in the profoundly coagulopathic patient with an active hemorrhage. Anticoagulation is a cornerstone of management for veno-arterial ECMO to prevent circuit thrombosis and systemic thromboembolism. But, we did not use heparin during this ECMO support. According to previous reports, in which mechanical circulatory support was used in patients with AFE, all patients could be weaned off the support within 2 to 3 days (10-13). So, we were planning to use heparin when the DIC and bleeding were controlled after the transfusion. Fortunately, in this case, the patient recovered rapidly the next day, and the ECMO was weaned successfully without a thromboembolic event.
In conclusion, AFE is an unpredictable life-threatening peripartum event. Recurrent cardiac arrest and severe DIC would lead to a dismal outcome, but patients can actually recover within a few days. Early and aggressive treatment, such as ECPR, is important and unique to save the life of both mother and the neonate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Society for Maternal-Fetal Medicine (SMFM), Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol 2016;215:B16-24. [Crossref] [PubMed]
- Creanga AA, Berg CJ, Syverson C, et al. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol 2015;125:5-12. [Crossref] [PubMed]
- Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, et al. Incidence, risk factors, management and outcomes of amniotic-fluid embolism: a population-based cohort and nested case-control study. BJOG 2016;123:100-9. [Crossref] [PubMed]
- Mhyre JM, Tsen LC, Einav S, et al. Cardiac arrest during hospitalization for delivery in the United States, 1998-2011. Anesthesiology 2014;120:810-8. [Crossref] [PubMed]
- Knight M, Tuffnell D, Brocklehurst P, et al. Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol 2010;115:910-7. [Crossref] [PubMed]
- Kramer MS, Rouleau J, Baskett TF, et al. Amniotic-fluid embolism and medical induction of labour: a retrospective, population-based cohort study. Lancet 2006;368:1444-8. [Crossref] [PubMed]
- Clark SL. Amniotic fluid embolism. Obstet Gynecol 2014;123:337-48. [Crossref] [PubMed]
- Huybrechts W, Jorens PG, Jacquemyn Y, et al. Amniotic fluid embolism: a rare cause of acute left-sided heart failure. Acta Cardiol 2006;61:643-9. [Crossref] [PubMed]
- Clark SL, Hankins GD, Dudley DA, et al. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol 1995;172:1158-67; discussion 1167-9. [Crossref] [PubMed]
- Ecker JL, Solt K, Fitzsimons MG, et al. Case records of the Massachusetts General Hospital. Case 40-2012. A 43-year-old woman with cardiorespiratory arrest after a cesarean section. N Engl J Med 2012;367:2528-36. [Crossref] [PubMed]
- Stanten RD, Iverson LI, Daugharty TM, et al. Amniotic fluid embolism causing catastrophic pulmonary vasoconstriction: diagnosis by transesophageal echocardiogram and treatment by cardiopulmonary bypass. Obstet Gynecol 2003;102:496-8. [PubMed]
- Hsieh YY, Chang CC, Li PC, et al. Successful application of extracorporeal membrane oxygenation and intra-aortic balloon counterpulsation as lifesaving therapy for a patient with amniotic fluid embolism. Am J Obstet Gynecol 2000;183:496-7. [Crossref] [PubMed]
- Lee JH, Jang HJ, Park JH, et al. Use of Extracorporeal Membrane Oxygenation in a Fulminant Course of Amniotic Fluid Embolism Syndrome Immediately after Cesarean Delivery. Korean J Crit Care Med 2016;31:256-61. [Crossref]


